May 16, 2013 — St. Jude Medical gained CE mark approval of its Ilumien Optis percutaneous coronary intervention (PCI) ...
FFR Technologies
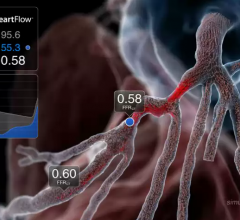
This channel includes news and new technology innovations for fractional flow reserve (FFR) wires, catheters and systems used to measure blood flow across a coronary lesion to determine if a stent is needed or if the plaque stenosis can be treated medically. The section includes iFR, instantaneous wave-free ratio, systems used in the cath lab and noninvasive FFR technologies including computed tomography-FFR. This is also referred to as CT-FFR or FFR-CT.


In the current era of healthcare reform and increasing requirements to follow appropriate use criteria, any technology ...
My job as editor of DAIC is to help readers keep tabs on the latest in cardiovascular technology and trends. With ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
My job as editor of DAIC is to help readers keep tabs on the latest in cardiovascular technology and trends. With ...
January 11, 2013 — Guided Interventions LLC, a startup company developing new, easier to use fractional flow reserve ...
December 26, 2012 — St. Jude Medical Inc. announced the first patient enrollment in its ILUMIEN I clinical study. The ...
The results of the FAME II Trial were presented at the 2012 Transcatheter Cardiovascular Therapeutics (TCT) meeting. The ...

Hospitals constantly try to predict what the future holds when planning new facilities and equipment purchases that will ...

October 30, 2012 — Fractional flow reserve (FFR)-guided treatment was shown to be cost effective for guiding coronary ...
September 17, 2012 — The Cardiovascular Research Foundation (CRF) announced the late-breaking trials and first report ...
September 11, 2012 — Data presented at the 2012 European Society of Cardiology (ESC) Congress in Munich, Germany, from ...

September 7, 2012 — St. Jude Medical Inc. announced results of the FAME II trial demonstrating that patients with fracti ...
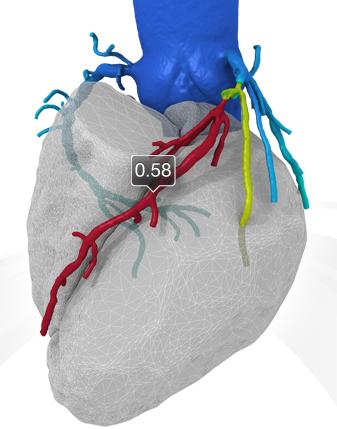
Trends and new technology for cardiac computed tomography angiography (CCTA) were highlighted during the 2012 Society of ...
May 24, 2012 — Volcano Corp. provided an overview of data from multiple ground-breaking clinical trials presented last ...
May 21, 2012 -- St. Jude Medical Inc. announced that data from the FAME II trial demonstrated a significant difference ...

 May 16, 2013
May 16, 2013