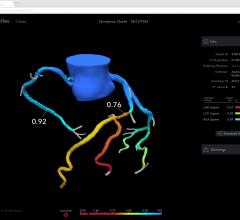
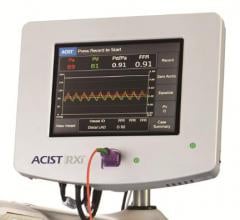
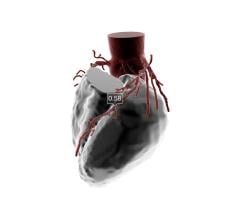
May 30, 2017 — Early results from the independent, physician-sponsored FFR-Search Registry revealed an association ...
FFR Technologies
This channel includes news and new technology innovations for fractional flow reserve (FFR) wires, catheters and systems used to measure blood flow across a coronary lesion to determine if a stent is needed or if the plaque stenosis can be treated medically. The section includes iFR, instantaneous wave-free ratio, systems used in the cath lab and noninvasive FFR technologies including computed tomography-FFR. This is also referred to as CT-FFR or FFR-CT.
MDBuyline analyst Tom Watson shares some of the most important trends in cardiac technology he saw at the 2017 American ...

March 27, 2017 — Patients experiencing a major heart attack often have more than one clogged artery, but under current ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
March 24, 2017 — At the 2017 American College of Cardiology's Annual Scientific Session & Expo (ACC.17), Philips ...
Justin Davies, MBBS, of Imperial College London, and Matthias Götberg, M.D, Ph.D., of Skane University Hospital, detail ...

March 20, 2017 — For patients experiencing angina (chest pain) or a heart attack, instantaneous wave-free ratio (iFR) ...
February 14, 2017 — The National Institute for Health and Care Excellence (NICE) in the United Kingdom recently issued g ...
January 12, 2017 — Acist Medical Systems Inc. announced that enrollment is complete for its Fractional Flow Reserve (FFR ...
DAIC Editor Dave Fornell takes a video tour of some of the most innovative new interventional cardiology technologies he ...
October 26, 2016 — Philips recently announced its latest image guidance solutions to be featured at the 2016 ...
October 10, 2016 — St. Jude Medical Inc. announced the U.S. clearance and launch of the company’s new PressureWire X ...
August 17, 2016 — Corindus Vascular Robotics Inc. and Acist Medical Systems Inc. are providing Fairview Southdale ...
June 29, 2016 — HeartFlow Inc. announced that it is launching its next generation of the HeartFlow FFR-CT Analysis. The ...
May 19, 2016 — St. Jude Medical Inc. announced results from two cardiovascular clinical trials presented at EuroPCR 2016 ...

April 28, 2016 — Abbott and St. Jude Medical Inc. announced a definitive agreement for Abbott to acquire St. Jude ...

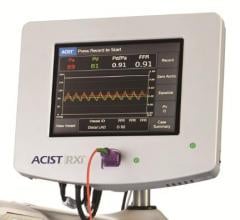
 May 30, 2017
May 30, 2017