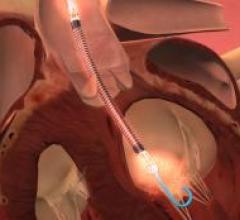
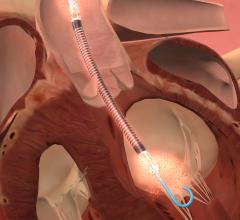
July 22, 2015 — St. Jude Medical and Thoratec announced that the boards of directors of both companies have unanimously ...
Hemodynamic Support Devices
This hemodynamic support systems channel includes content on intra-aortic balloon pumps (IABP), percutaneous ventricular assist devices (pVAD) like the Impella or TandemHeart, extracorporeal membrane oxygenation (ECMO), and ventricular assist devices (VAD). This channel also includes use of these devices in support of patients in cardiogenic shock and advanced heart failure.
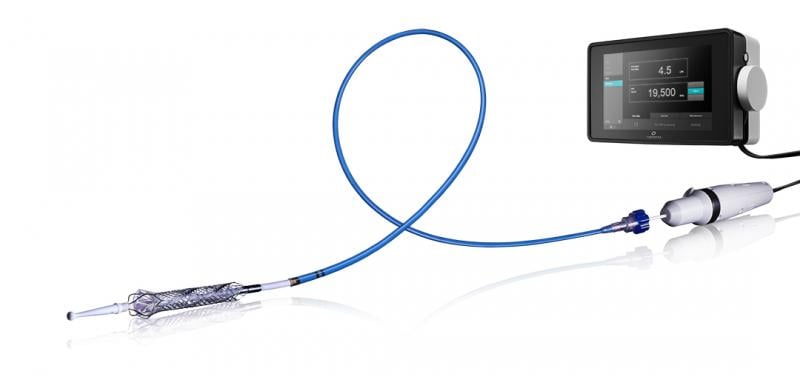
July 15, 2015 - Thoratec announced that the HeartMate PHP (Percutaneous Heart Pump) received CE Mark approval ...
July 9, 2015 - During an event with Massachusetts Gov. Charlie Baker in June, Abiomed announced a major expansion of its ...
June 11, 2015 - ECRI Institute recently reviewed published data on Abiomed's Impella RP (Right Percutaneous) ...
June 9, 2015 - University Hospitals Case Medical Center physicians in the Harrington Heart & Vascular Institute were the ...
June 4, 2015 - Beaumont Hospital, Royal Oak is one of the first centers in the United States to use a new minimally ...

June 1, 2015 — Maquet Getinge Group announced the publication of a manuscript describing the exploration of the ...
May 4, 2015 — Thoratec Corp. announced results from the ROADMAP Study (Risk Assessment and Comparative Effectiveness of ...
April 27, 2015 — CardiacAssist Inc. announced that it received a Class 3 CE-mark for its Protek Duo veno-venous cannula ...
April 9, 2015 — Maquet Cardiovascular USA announced publication of a manuscript comparing the clinical and economic ...
April 7, 2015 — An expert consensus statement released by four leading cardiovascular societies provides new guidance on ...
April 2, 2015 — SynCardia Systems Inc. has received U.S. Food and Drug Administration (FDA) approval to conduct an ...
March 24, 2015 – The U.S. Food and Drug Administration (FDA) has granted pre-market approval (PMA) clearance for Abiomed ...
January 30, 2015 — SynCardia Systems Inc. has received U.S. Food and Drug Administration (FDA) approval to conduct a cli ...
January 27, 2015 — NewYork-Presbyterian Hospital/Columbia University Medical Center is one of a select group of medical ...


 July 22, 2015
July 22, 2015