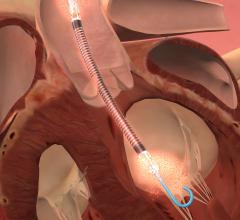
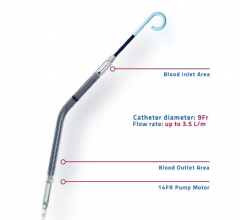
This video, provided by Abiomed, demonstrates the Impella percutaneous ventricular assist devices (pVAD). It offers ...
Hemodynamic Support Devices
This hemodynamic support systems channel includes content on intra-aortic balloon pumps (IABP), percutaneous ventricular assist devices (pVAD) like the Impella or TandemHeart, extracorporeal membrane oxygenation (ECMO), and ventricular assist devices (VAD). This channel also includes use of these devices in support of patients in cardiogenic shock and advanced heart failure.
March 9, 2017 — Metro Detroit cardiologists from five health systems have joined together to increase residents’ ...
March 9, 2017 — A new study published in Circulation Research finds use of hemodynamic support with the Impella 2.5 hear ...
March 8, 2017 — Congestive heart failure patients at the University of Alabama at Birmingham (UAB) now have reason for ...
February 24, 2017 — Abiomed Inc. announced that it has supported more than 50,000 patients in the U.S. with its Impella ...
January 31, 2017 — To save the life of an 11-year-old boy, Ann & Robert H. Lurie Children’s Hospital of Chicago has ...
January 17, 2017 — Investigators at the University of Utah have identified distinct differences in the hearts of ...
January 3, 2017 — The Impella CP heart pump (Abiomed) demonstrated no improvement in mortality for patients with ...

The capsule endoscopy systems offer a hands-free and highly accurate imaging modality for healthcare practitioners. A ...
December 8, 2016 — St. Jude Medical Inc. announced results of the MOMENTUM 3 U.S. IDE Clinical Study during a late ...
December 7, 2016 — Abiomed Inc. announced it has expanded its U.S. Food and Drug Administration (FDA) pre-market ...
October 27, 2016 — Abiomed Inc. said the U.S. Food and Drug Administration (FDA)gave approval for a prospective ...
October 26, 2016 — The U.S. Food and Drug Administration (FDA) provided an update and additional information regarding ...
October 5, 2016 — Medtronic said two previously communicated global voluntary recalls related to the HeartWare ...

New cardiovascular device therapies for atrial fibrillation (AF) and heart failure (HF) are rapidly evolving with the ...


 March 21, 2017
March 21, 2017