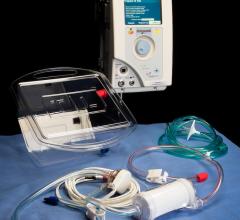
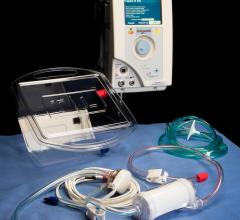
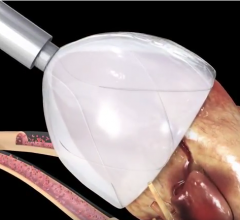
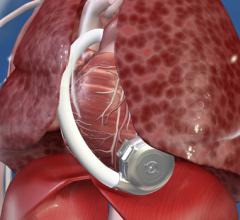
February 22, 2018 — When someone goes into cardiac arrest and first responders cannot shock their heart back into rhythm ...
Hemodynamic Support Devices
This hemodynamic support systems channel includes content on intra-aortic balloon pumps (IABP), percutaneous ventricular assist devices (pVAD) like the Impella or TandemHeart, extracorporeal membrane oxygenation (ECMO), and ventricular assist devices (VAD). This channel also includes use of these devices in support of patients in cardiogenic shock and advanced heart failure.
February 15, 2018 — Abiomed Inc. announced that it received an expanded U.S. Food and Drug Administration (FDA) Pre ...
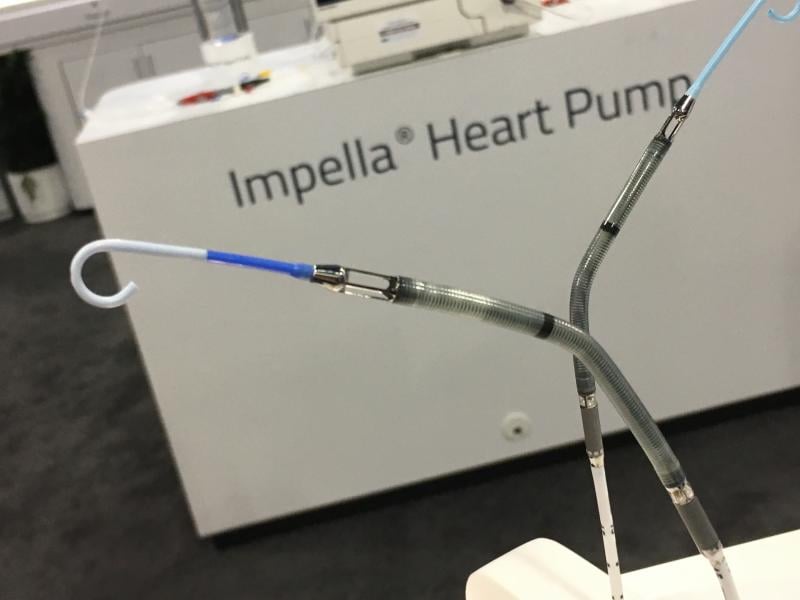
February 14, 2018 — Abiomed Inc. announced it received an expanded U.S. Food and Drug Administration (FDA) pre-market ...
January 25, 2018 – According to the latest market study released by Technavio, the global circulatory support devices ...
January 16, 2018 — Miracor Medical Systems GmbH (Miracor Austria) and Miracor Medical SA (Miracor Medical) announced the ...
December 19, 2017 — A team of researchers at the National Institute for Health Research (NIHR) Leicester Biomedical ...
December 11, 2017 — CorInnova Inc. recently announced it was awarded second prize in the “2017 InnoSTARS” life science ...
November 17, 2017 — Common practice, and recently published research, shows that the risk of complications with surgery ...
DAIC Editor Dave Fornell shows some of the innovations displayed on the expo floor at the 2017 Transcatheter ...
A discussion with William W. O’Neill, M.D., medical director, Center for Structural Heart Disease, Henry Ford Hospital ...
October 27, 2017 — New Milford, Conn., resident Neta Awasthi is thankful to be home with his wife, Rhicha, and two ...
October 27, 2017 — CorInnova Inc. announced it has received notice of allowance of a seminal patent to protect its ...
October 26, 2017 — Medical professionals representing hospital systems from across the United States are expected to ...
October 4, 2017 — Medtronic received U.S. Food and Drug Administration (FDA) approval for its HeartWare HVAD System as a ...
September 28, 2017 — Abiomed Inc. recently received U.S. Food and Drug Administration (FDA) pre-market approval (PMA) ...

 February 22, 2018
February 22, 2018