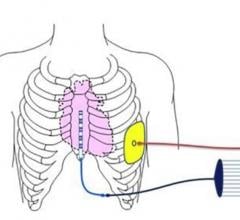
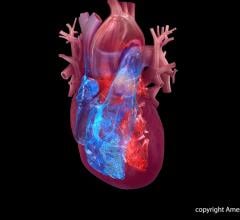
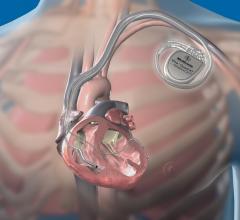
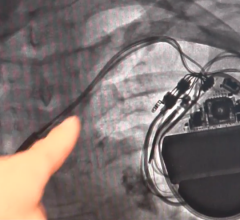
August 5, 2019 — Abbott is recalling the Ellipse Implantable Cardioverter Defibrillators (ICDs) because electrical ...
Leads Implantable Devices
News and new technology innovations for leads implantable devices can be found on this channel.
May 13, 2019 — BioTrace Medical Inc. announced the company’s Tempo Lead has obtained CE Mark certification in Europe for ...
May 6, 2019 — Medtronic has received U.S. Food and Drug Administration (FDA) approval for the Attain Stability Quad MRI ...
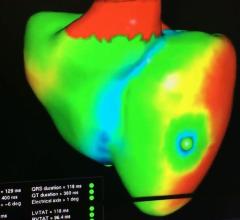
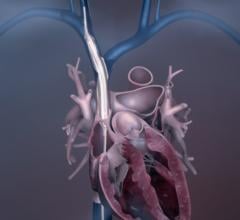
This is a virtual heart with the same electrophysiology characteristics as the real patient being developed to help ...
Interventional cardiac resynchronization therapy (I-CRT) describes the repurposing of a set of tools and techniques ...

To extract or abandon broken or infected implantable, venous electrophysiology (EP) device leads has been a debate for ...
August 9, 2018 — Medtronic plc announced the start of a pilot study of its investigational Extravascular Implantable ...
May 24, 2018 - Medtronic plc announced results from a research study demonstrating the feasibility of a novel approach ...
May 18, 2018 — Data on the effectiveness of cardiac resynchronization therapy (CRT) in patients with non-left bundle ...
May 10, 2018 – A new study is the first to test the clinical effectiveness of incremental peri-operative antibiotics as ...
March 6, 2018 – Nihon Kohden announced the launch of the Nihon Kohden Dimensions Augmented Reality (AR) App to ...
September 27, 2017 — Spectranetics is recalling its Bridge Occlusion Balloon Catheter due to the possibility of a ...

Electrophysiology (EP) technology has been advancing rapidly the past few years with new ablation tools to improve ...
June 20, 2017 — BioTrace Medical Inc. announced that the company’s Tempo Temporary Pacing Lead was featured in an oral ...
DAIC Editor Dave Fornell takes a tour of some of the most innovative new electrophysiology (EP) technology at the 2017 H ...

 August 05, 2019
August 05, 2019