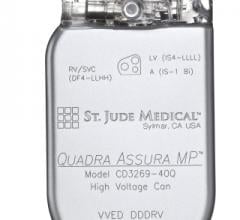
March 28, 2014 — St. Jude Medical Inc. announced the global launch of the Optisure defibrillation lead. This new lead is ...
Leads Implantable Devices
News and new technology innovations for leads implantable devices can be found on this channel.
March 24, 2014 — Sorin Group has purchased Oscor Inc. lead business, including a lead manufacturing facility in the ...
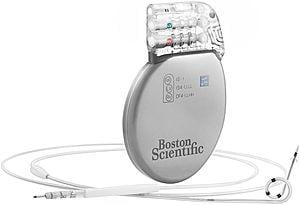
March 21, 2014 — Boston Scientific Corp. received CE marking and launched in Europe the Ingevity family of magnetic ...
March 6, 2014 — Biotronik announced the first implantations of the Sentus ProMRI lead. Biotronik’s bipolar cardiac ...


June 6, 2013 — Data presented at Heart Rhythm 2013 shows that Medtronic Inc. Lead Integrity Alert (LIA) software ...
May 13, 2013 — The Population Health Research Institute (PHRI) has conducted a further independent analysis of data ...
May 6, 2013 — St. Jude Medical announced its first enrollment in its MultiPoint Pacing clinical study to build upon its ...
April 30, 2013 — St. Jude Medical Inc. announced CE Mark approval and European launch of its Allure Quadra Cardiac ...
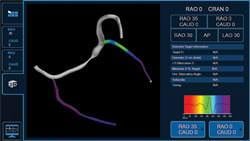
April 10, 2013 — Medtronic Inc. announced market release of the CardioGuide Implant System, a novel real-time navigation ...
March 18, 2013 — Medtronic Inc. announced it has received CE mark and will begin the European launch of the Attain ...
February 25, 2013 — The U.S. Food and Drug Administration (FDA) granted final approval for the Biotronik Lumax 740 DX ...
December 28, 2012 — The first patient has been implanted with the Boston Scientific Corporation next generation Ingevity ...


 March 28, 2014
March 28, 2014