August 15, 2014 — OrbusNeich announced first patient enrollment in the Randomized Evaluation of short-term Dual anti ...
Stents Drug Eluting
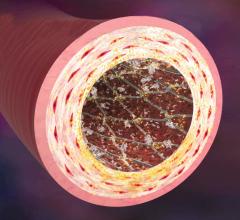
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
July 30, 2014 — OrbusNeich announced the first patient has been enrolled in the Multinational Abluminal Sirolimus Coated ...
July 15, 2014 — Micell Technologies Inc. announced the United States Patent and Trademark Office (USPTO) has issued a ...
A big fly in the ointment for widespread adoption of many new technologies is cost. In today’s cost-conscience ...
A big fly in the ointment for widespread adoption of many new technologies is cost. In today’s cost-conscience ...
June 24, 2014 — OrbusNeich announced the completion of enrollment in the prospective, multicenter, all-comers REMEDEE ...
June 10, 2014 — Ariad Pharmaceuticals Inc. and Medinol Ltd. earlier this year initiated two registration trials of ...
May 1, 2014 — Boston Scientific Corp. has launched the Promus Premier Everolimus-Eluting Platinum Chromium Coronary ...
April 4, 2014 — A new stent covered with biodegradable coating continues to show statistical equivalence to Japan’s ...
March 18, 2014 — Patients with coronary artery disease who received an Endeavor or Resolute drug-eluting stent from ...
By Dave Fornell, DAIC editor
The Cardiovascular Research Foundation’s Transcatheter Cardiovascular Therapeutics (TCT) ...
November 1, 2013 – In the first head-to-head randomized controlled trial of third-generation durable-polymer drug ...


 August 15, 2014
August 15, 2014