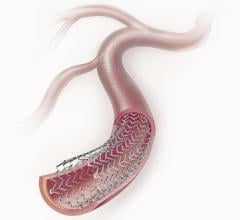
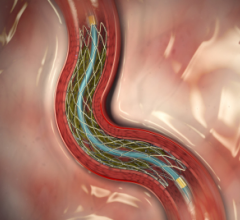
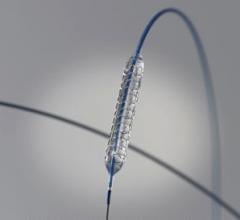
March 29, 2012 — In the second year of an ongoing trial, the Resolute zotarolimus-eluting stent (Medtronic) achieved a ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
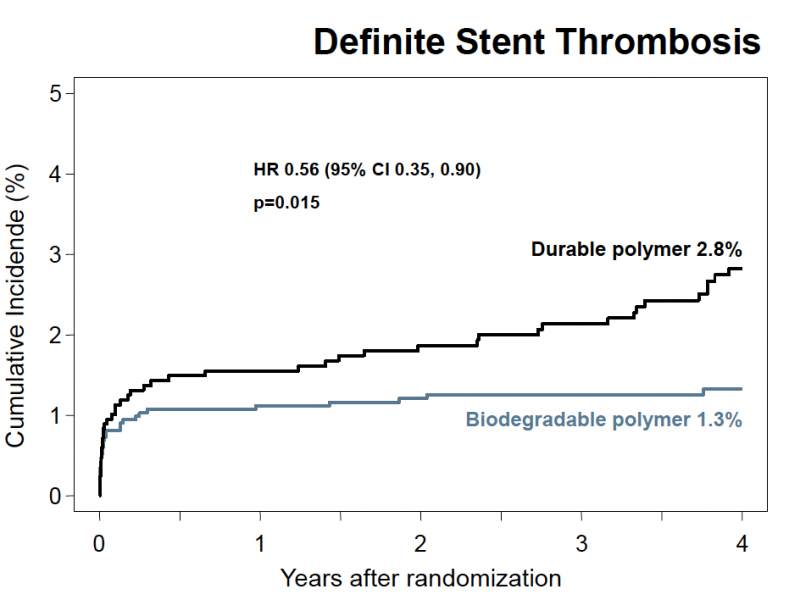
March 27, 2012 — Biodegradable polymer drug-eluting stents (DES) provide better long-term safety and efficacy than ...

April 15, 2012, will mark the 10th anniversary of the approval of the first drug-eluting stent (DES). Ten years ago, the ...
March 9, 2012 — Boston Scientific Corp. announced the launch of the Promus Element everolimus-eluting coronary stent sys ...
March 6, 2012 — In a development that brings advanced combination therapy treatment of peripheral artery disease (PAD) t ...
February 24, 2012 – The U.S. Food and Drug Administration (FDA) this week granted the first coronary stent indication ...
February 20, 2012 — Medtronic announced U.S. Food and Drug Administration (FDA) approval of the Resolute Integrity drug ...
February 1, 2012 — Boston Scientific announced the first patient use and European market launch of the Promus Element ...
January 23, 2012 – Boston Scientific Corp. said the U.S. District Court for the District of New Jersey has found all the ...
January 9, 2012 – A registry that includes every patient in Sweden having percutaneous coronary intervention (PCI) found ...
December 22, 2011 – Abbott today announced the initiation of ESPRIT I, a first-of-its-kind clinical trial in Europe ...
December 12, 2011 - Boston Scientific recently announced U.S. Food and Drug Administration (FDA) approval for the Promus ...
December 9, 2011 — Abbott announced Thursday the initiation of ABSORB II, the first randomized, controlled, multi-center ...
November 21, 2011 — Micell Technologies Inc. announced the release of preliminary data from the first-in-human clinical ...
November 15, 2011 – The risk of late stent thrombosis (ST) in the first generation of drug-eluting stents continues for ...

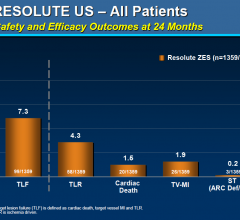
 March 29, 2012
March 29, 2012