The anti-proliferative drug paclitaxel has been used as a coating on coronary stents to prevent restenosis since 2003 ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
The rise cardiovascular disease has been instrumental in fueling the coronary stent market share in the past few years ...
January 17, 2019 — The U.S. Food and Drug Administration (FDA) issued a letter Jan. 17, 2019, to healthcare providers ...
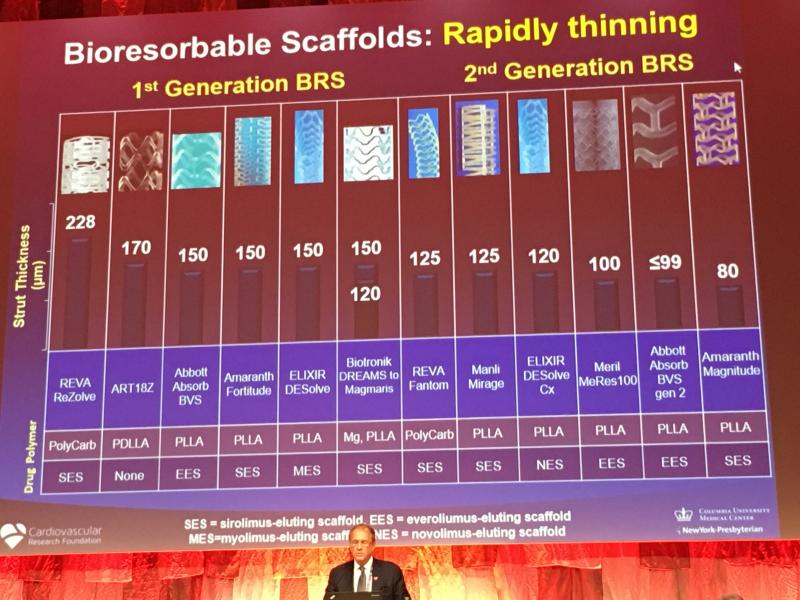
Bioresorbable stent (BRS) technology is not dead, but the unbridled enthusiasm seen two years ago for the technology has ...
Professor Ian Meredith, MBBS, Ph.D., global chief medical officer and executive vice president, Boston Scientific ...
A discussion with Professor Ian Meredith, MBBS, Ph.D., global chief medical officer and executive vice president, Boston ...
October 4, 2018 – Investigators unveiled clinical data from the independent BIONYX and SORT OUT IX all-comers trials ...
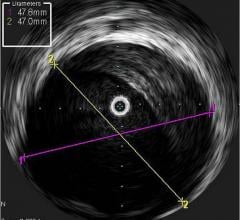
October 2, 2018 — Intravascular ultrasound (IVUS) guidance improved clinical outcomes over angiography guidance during d ...
October 1, 2018 — Recent results from the BIONYX randomized clinical study showed the novel, thin-strutted, polymer ...
October 1, 2018 — Boston Scientific announced that the U.S. Food and Drug Administration (FDA) has approved its ...
September 26, 2018 — Positive 12-month data from the late-breaking IMPERIAL trial was presented at the 2018 ...
May 31, 2018 – Late-breaking trial results presented at the EuroPCR Congress, May 21-24 in Paris, France, found the ...
May 31, 2018 — Two-year outcome data from the BIO-RESORT randomized controlled trial were presented in a late-breaking ...
May 29, 2018 – Investigators recently unveiled clinical data from the independently run Onyx 1-Month OCT Study showing ...
May 29, 2018 – Medtronic plc announced the initiation of a U.S. clinical study to assess the safety and efficacy of drug ...

 January 25, 2019
January 25, 2019