October 17, 2023 — Scientists from The Florey are among the world’s leading stroke experts who have mapped out how ...
Stroke
This channel includes news and new technology innovations for stroke. It includes both diagnosis and treatment of stroke, stroke imaging, pharmaceuticals and interventional stroke technologies. Stroke comes in two forms, which have different therapies.
1. Ischemic stoke is a blockage of an artery in the brain, preventing blood flow and is offen referred to as a "brain attack" because it is a similar casue as a heart attack. This type of stroke is often treated with anti-coagulants, including use of tissue plasminogen activator (tPA). Interventional mechanical thrombectomy can also be used to remove the clot.
2. Hemorrhagic stroke is caused when there is bleeding due to a ruptured blood vessel in the brain caused by a brain aneurysm burst or a weakened blood vessels. These strokes are less common, but exact diagnosis is important, because use of tPA in these patients can have catastrophic consequences. Treatments include interventional embolization and surgical clipping of target vessels.
October 12, 2023 — Royal Philips, a global leader in health technology, and the World Stroke Organization (WSO), the ...

The global incidence of non-communicable diseases (NCDs) is currently around 40 million cases a year. Of that total ...
October 3, 2023 — A discovery of a mutation in the gene ACTA2 has given researchers, led by Dianna Milewicz, MD, PhD ...
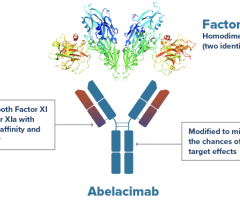
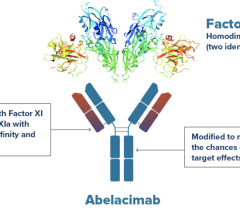
September 26, 2023 — Anthos Therapeutics, Inc., a clinical stage company developing innovative therapies for ...
September 22, 2023 — For many patients with hypertension--an elevated blood pressure that can lead to stroke or heart ...
September 18, 2023 — Pregnant women with a history of substance abuse face a dramatically increased risk of death from ...
September 18, 2023 — Anthos Therapeutics, Inc., a clinical stage company developing innovative therapies for cardiovascu ...
September 15, 2023 — Suffering a traumatic brain injury (TBI) – no matter how severe – is associated with a ...
September 7, 2023 — Boston Scientific Corporation announced it has received U.S. Food and Drug Administration approval ...
September 5, 2023 — Cedars-Sinai investigators are leaders in the innovation and use of transcatheter aortic valve ...
August 22, 2023 — Heart attack patients who do not take daily aspirin have an elevated likelihood of recurrent myocardia ...
August 22, 2023 — A study in more than 15,000 people has found that physical fitness is linked with a lower likelihood ...
August 22, 2023 — Recognizing and acting on heart attack symptoms is linked with faster life-saving treatment, according ...
August 17, 2023 — University of Missouri School of Medicine neurologist Adnan Qureshi, MD recently led a study that ...

 October 17, 2023
October 17, 2023