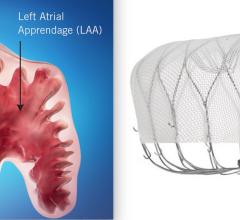
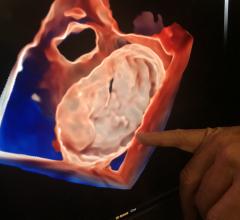
January 17, 2022 – As the increasing number of structural heart interventions are assisted by real-time imaging guidance ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
December 13, 2021 - Foldax Inc. announced that the Drugs Controller General of India (DCGI), India’s regulatory body for ...
Examples of TrueView and GlassView 3D cardiac ultrasound visualization and artificial intelligence (AI) assisted ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
November 22, 2021 — Atlantic Health System’s Morristown Medical Center announced that it has enrolled the first patient ...
Christine Albert, M.D., MPH, professor, chair of the Department of Cardiology and the Lee and Harold Kapelovitz ...
November 18, 2021 — Adults undergoing mitral valve surgery who have less than severe leakage of the tricuspid valve may ...
November 17, 2021 — For people experiencing severe aortic stenosis who do not have symptoms or need symptom relief ...
November 10, 2021 — Medtronic presented early data for its self-expanding Intrepid transcatheter mitral valve ...
November 10, 2021 — Ancora Heart Inc., a company developing a novel therapy to address heart failure, announced results ...
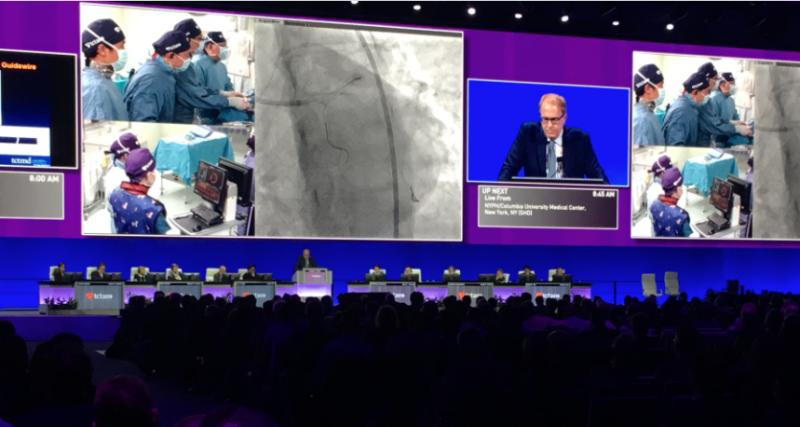
November 10, 2021 — There were nine late-breaking trials and 13 late-breaking science presentations at the 2021 Transcat ...
November 9, 2021 — Results from the largest randomized trial available comparing different closure device strategies ...
November 9, 2021 — New five-year data from the SURTAVI trial found that there was no difference in all-cause mortality ...
November 9, 2021 — An economic analysis of data from PARTNER 3, a randomized trial comparing transcatheter aortic valve ...
November 8, 2021 – SWISS-APERO is the first randomized clinical trial comparing the Abbott Amulet left atrial appendage ...
November 8, 2021 — Results from a clinical trial of the Edwards Lifesciences Evoque transcatheter tricuspid valve ...

 January 17, 2022
January 17, 2022