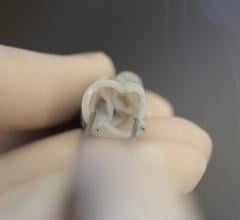
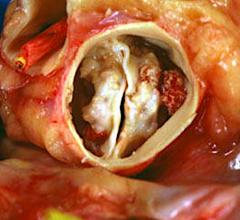
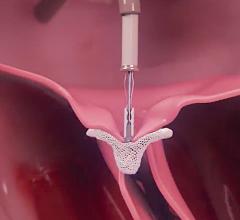
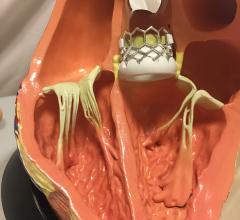
Interview with Scott E. Kasner, M.D., who served as the principle investigator for the Gore Cardioform REDUCE trial, and ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
March 18, 2020 — A groundbreaking new study led by University of Minnesota Twin Cities researchers from both the College ...

While many cardiac and vascular procedures have largely moved to minimally invasive techniques, the size of these ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

The latest cardiology practice-changing scientific breakthrough, late-breaking study presentations have been announced ...
February 24, 2021 — Occlutech announced the completion of patient enrollment in the PRELIEVE trial, pilot study to ...
February 10, 2021 - The U.S. Food and Drug Administration (FDA) has granted artificial heart developer Carmat approval ...
February 4, 2021 — Transcatheter aortic valve replacement (TAVR) has historically only been used with caution in the ...
January, 27, 2021 — The U.S. Food and Drug Administration (FDA) has granted Occlutech a Breakthrough Device designation ...
January 20, 2021 — The U.S. Centers for Medicare and Medicaid Services (CMS) revised its National Coverage Determination ...
January 11, 2021 — Medtronic has received a new expanded indication from Health Canada for its Evolut Transcatheter ...
January 6, 2021 — The U.S. Food and Drug Administration (FDA) has granted the designation of breakthrough device to P+F ...
January 5, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
December 29, 2020 — Edwards Lifesciences announced Dec. 21 that the first patient was treated in the RESTORE clinical ...
December 29, 2020 — Health Canada has approved the expanded use of the Edwards Lifesciences Sapien 3 and Sapien 3 Ultra ...
Here are the top 25 best performing articles on the Diagnostic and Interventional Cardiology (DAIC) website from 2020 ...


 March 23, 2021
March 23, 2021