(This article was updated in June 2018)
The advent of transcatheter valve repair and replacement technologies is one ...
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
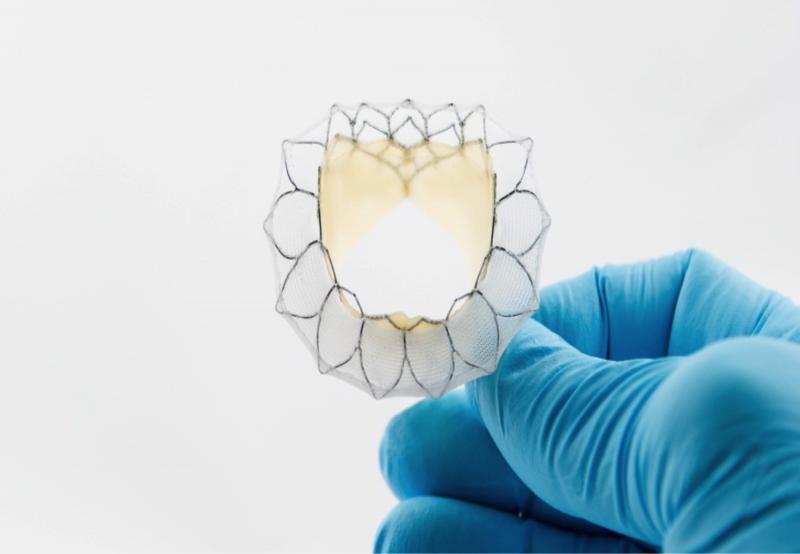
(This article was updated in June 2018)
The advent of transcatheter valve repair and replacement technologies is one ...
November 10, 2015 — PinnacleHealth CardioVascular Institute enrolled the first patient in Pennsylvania in a randomized ...
For the past few years, the focus of the annual Transcatheter Cardiovascular Therapeutics (TCT) meetings has been on ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
DAIC Editor Dave Fornell offers his choices for the most innovative new interventional cardiovascular technologies ...
Tom Watson, clinical analyst for MDBuyLine, and DAIC Editor Dave Fornell discuss some of the new cardiovascular and ...
October 28, 2015 — SentreHeart Inc. announced that it has received CE Mark approval for the Lariat Surgical Left Atrial ...
October 27, 2015 — A large observational registry found that initial aortic valve replacement (AVR) in asymptomatic ...
October 22, 2015 — Medtronic plc released the first CoreValve transcatheter aortic valve replacement (TAVR) system outco ...
October 22, 2015 — Direct Flow Medical Inc. announced one-year outcomes from the DISCOVER post-market study that ...

October 20, 2015 — Results from the BRAVO 3 trial found that bivalirudin did not significantly reduce major bleeding ...
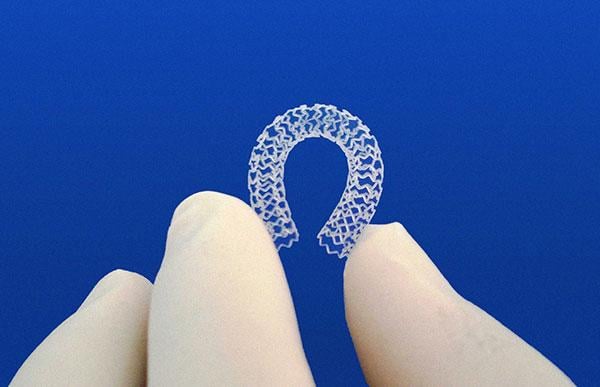
There were several overarching technology trends seen at the 2015 Transcatheter Cardiovascular Therapeutics (TCT) annual ...
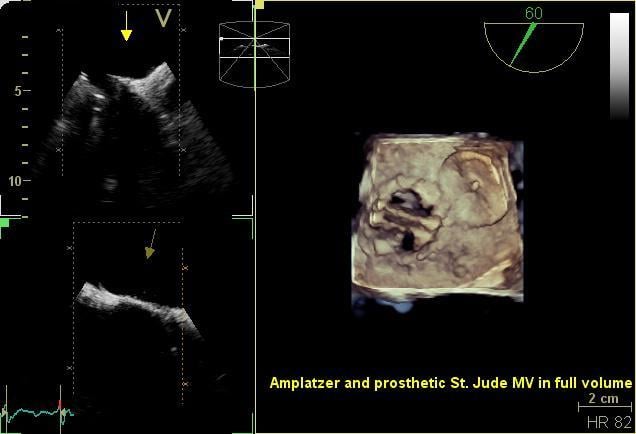
October 16, 2015 — Long-term study results from the RESPECT trial found that closing a patent foramen ovale (PFO) with ...
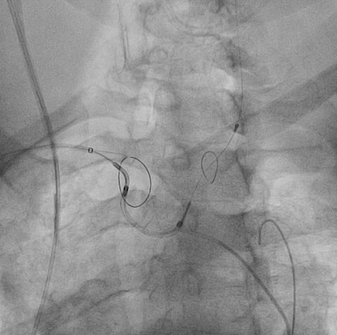
October 14, 2015 — Claret Medical, an innovator in cardiovascular cerebral protection, Data presented this week at ...
October 15, 2015 — Edwards Lifesciences Corp. announced that details on the 13 first-in-human compassionate use cases wi ...

October 15, 2015 — One-year patient outcomes from the PARTNER II trial showed the low rate of 30-day complications with ...