Feb. 2, 2026 — Brainomix, a provider of AI-powered imaging tools in stroke and lung fibrosis, is introducing its new Brainomix 360 Next Generation platform at the International Stroke Conference (ISC) ...
Thrombectomy Devices
This channel includes news and new technology innovations for catheter-based thrombectomy systems used to remove blood clots from vessels in the body. Thrombus removal a decade ago was a standard of care for acute coronary revascularization in ST-elevation myocardial infarction (STEMI). But, its use declined rapidly after several large trials showed no benefit. However today, thrombectomy is seeing increasing usage in new therapeutic areas to treat acute stoke, pulmonary embolism (PE), deep vein thrombosis (DVT) and venous thromboembolism (VTE). Thrombectomy is also referred to as embolectomy.
The types of thrombectomy systems include:
● Ultrasound-assisted thrombolysis – Catheter-directed, high-frequency ultrasound helps thrombolytic agents penetrate clots to speed the action of fibrinolytic pharmacological therapy.
● Rheolytic embolectomy – These devices inject pressurized saline through the catheter's distal tip and the macerated thrombus is aspirated through a catheter port.
● Rotational embolectomy – A rotating device at the catheter tip is used to fragment the clot and fragments are aspirated by the catheter.
● Aspiration thrombectomy – This includes manual clot aspiration or use of dedicated aspiration catheter devices that basically vacuum the clot out of the vessel.
● Thrombus fragmentation – Thrombus can be mechanically disrupted by manually rotating a pigtail catheter or using balloon angioplasty, but this causes small fragments of emboli to flow distally. Dedicated devices also are available.
Feb. 2, 2026 — Brainomix, a provider of AI-powered imaging tools in stroke and lung fibrosis, is introducing its new ...
Jan. 28, 2026 — Imperative Care has announced the commercial launch and first patient cases of the new Zoom 4S Catheter ...
Jan. 15, 2026 — Boston Scientific Corp. and Penumbra, Inc. have entered into a definitive agreement under which Boston ...
Dec. 10, 2025 — Medtronic plc has announced the first commercial use of the Liberant thrombectomy system (Liberant) ...
Dec. 16, 2025 — A new study, published in the American Journal of Cardiology, found that the use of Computer Assisted ...
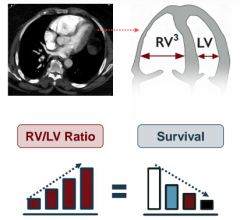
Nov. 3, 2025 — Penumbra, Inc. has announced additional results of the STORM-PE randomized controlled trial (RCT), which ...
Oct. 27, 2025 – Penumbra, Inc. has announced the results of the STORM-PE randomized controlled trial (RCT), which found ...
Sept. 2, 2025 — Imperative Care, Inc. has announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its ...
June 16, 2025 – Penumbra, Inc. recently announced the completion of enrollment in the STORM-PE clinical trial. This ...
March 24, 2025 — Imperative Care, Inc. recently announced U.S. Food and Drug Administration (FDA) 510(k) clearance of ...
Penumbra recently launched its Element Vascular Access System, a laser-cut hypotube sheath designed for venous ...
Nov. 5, 2024 —Penumbra, Inc. recently announced new data that demonstrate patients with intermediate-risk pulmonary ...
Sept. 24, 2024 — Thrombolytic Science, LLC (TSI) has announced that the U.S. Food and Drug Administration (FDA) has ...
Sept. 5, 2024 - Prolocor, Inc. and Slingshot Biosciences recently announced the publication in Bioanalysis of the ...
Sept. 10, 2024 — Royal Philips and the World Stroke Organization (WSO) have published a policy paper calling for a ...




 February 02, 2026
February 02, 2026