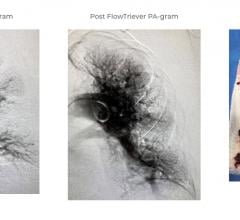
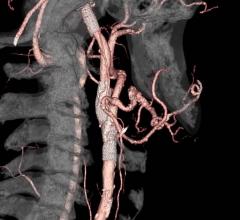
January 8, 2021 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the Inari Medical Inc ...
Thrombectomy Devices
This channel includes news and new technology innovations for catheter-based thrombectomy systems used to remove blood clots from vessels in the body. Thrombus removal a decade ago was a standard of care for acute coronary revascularization in ST-elevation myocardial infarction (STEMI). But, its use declined rapidly after several large trials showed no benefit. However today, thrombectomy is seeing increasing usage in new therapeutic areas to treat acute stoke, pulmonary embolism (PE), deep vein thrombosis (DVT) and venous thromboembolism (VTE). Thrombectomy is also referred to as embolectomy.
The types of thrombectomy systems include:
● Ultrasound-assisted thrombolysis – Catheter-directed, high-frequency ultrasound helps thrombolytic agents penetrate clots to speed the action of fibrinolytic pharmacological therapy.
● Rheolytic embolectomy – These devices inject pressurized saline through the catheter's distal tip and the macerated thrombus is aspirated through a catheter port.
● Rotational embolectomy – A rotating device at the catheter tip is used to fragment the clot and fragments are aspirated by the catheter.
● Aspiration thrombectomy – This includes manual clot aspiration or use of dedicated aspiration catheter devices that basically vacuum the clot out of the vessel.
● Thrombus fragmentation – Thrombus can be mechanically disrupted by manually rotating a pigtail catheter or using balloon angioplasty, but this causes small fragments of emboli to flow distally. Dedicated devices also are available.
October 23, 2020 — Positive results were reported on the first 230 patients enrolled in its FLASH study, a real world p ...
September 25, 2020 — Philips Healthcare launched its QuickClear mechanical thrombectomy system. The compact, single-use ...
July 20, 2020 – BD (Becton, Dickinson and Company) recently completed the acquisition of Straub Medical AG, a privately ...
November 7, 2019 — The ClotTriever Outcomes (CLOUT) Registry is evaluating real-world patient outcomes following ...
November 7, 2019 — The REVEAL FDA investigational device exemption (IDE) trial confirmed a favorable safety and ...
November 8, 2019 — Penumbra Inc. announced that the EXTRACT-PE trial successfully met the primary endpoints ...
October 18, 2019 — An algorithm developed by faculty at The University of Texas Health Science Center at Houston ...
July 25, 2019 — Penumbra announced U.S. commercial availability of the Penumbra System’s most advanced technology, the ...
March 27, 2019 — Training interventional radiologists to perform endovascular thrombectomies results in positive ...
February 12, 2019 — A study presented at the 2018 annual meeting of the Cardiovascular and Interventional Radiology ...
February 5, 2019 — Stroke survivors have better quality of life three months after their stroke if the clot that caused ...

Catheter-based blood clot removal a decade ago was a standard of care for acute coronary revascularization, but declined ...
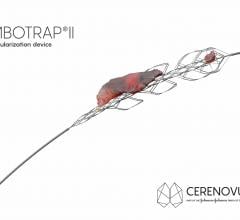
January 16, 2019 — Cerenovus, of Johnson & Johnson Medical Devices Companies, recently launched the EXCELLENT Registry ...
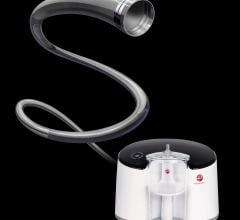
November 21, 2018 — BTG plc announced the first Ekos CU 4.0 units have been shipped from BTG’s facility in Bothell, Wash ...

 January 08, 2021
January 08, 2021