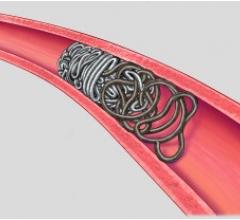
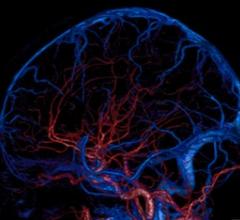
March 8, 2016 — phenox GmbH announced participation in the SITS Open clinical trial, where pREset and pREset LITE thromb ...
Thrombectomy Devices
This channel includes news and new technology innovations for catheter-based thrombectomy systems used to remove blood clots from vessels in the body. Thrombus removal a decade ago was a standard of care for acute coronary revascularization in ST-elevation myocardial infarction (STEMI). But, its use declined rapidly after several large trials showed no benefit. However today, thrombectomy is seeing increasing usage in new therapeutic areas to treat acute stoke, pulmonary embolism (PE), deep vein thrombosis (DVT) and venous thromboembolism (VTE). Thrombectomy is also referred to as embolectomy.
The types of thrombectomy systems include:
● Ultrasound-assisted thrombolysis – Catheter-directed, high-frequency ultrasound helps thrombolytic agents penetrate clots to speed the action of fibrinolytic pharmacological therapy.
● Rheolytic embolectomy – These devices inject pressurized saline through the catheter's distal tip and the macerated thrombus is aspirated through a catheter port.
● Rotational embolectomy – A rotating device at the catheter tip is used to fragment the clot and fragments are aspirated by the catheter.
● Aspiration thrombectomy – This includes manual clot aspiration or use of dedicated aspiration catheter devices that basically vacuum the clot out of the vessel.
● Thrombus fragmentation – Thrombus can be mechanically disrupted by manually rotating a pigtail catheter or using balloon angioplasty, but this causes small fragments of emboli to flow distally. Dedicated devices also are available.
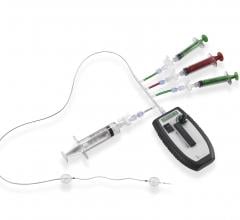
January 14, 2016 — Penumbra Inc. announced the U.S. launch of its new POD Packing Coil, designed as a complementary ...
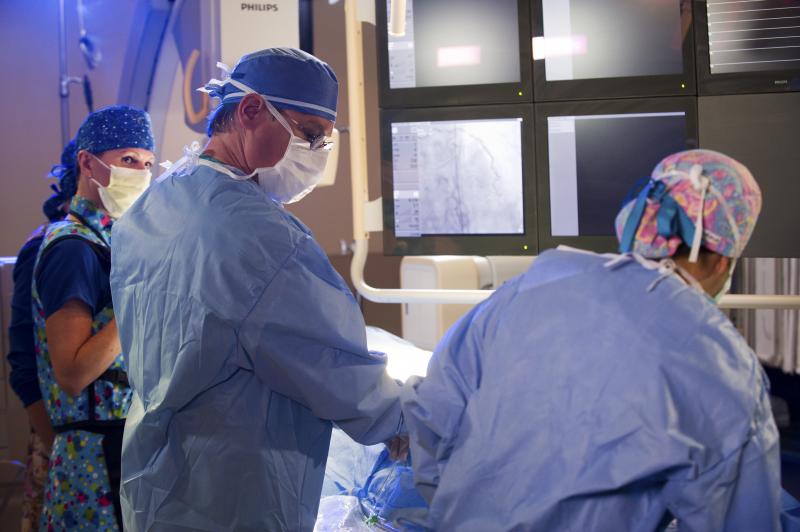
October 20, 2015 — A pair of studies presented at the 27th annual Transcatheter Cardiovascular Therapeutics (TCT) ...
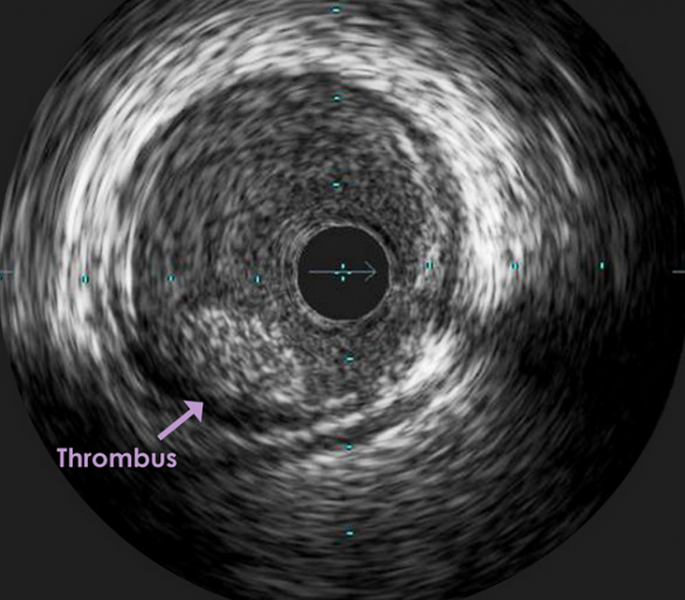
September 2, 2015 — Retrieval of larger thrombi during intra-arterial treatment (IAT) is associated with improved ...
Interview at the American Society of Echocardiography (ASE) annual meeting with Federico Asch, M.D., M.D., FACC, FASE ...

June 12, 2015 - Researchers presented data at the 2015 American Society of Echocardiography (ASE) meeting in June ...
June 11, 2015 - C. Dorn Smith, M.D., vascular surgeon in Kingstree, South Carolina, was successful in using a new device ...
June 5, 2015 - Penumbra Inc. announced that the company's ACE64 aspiration thrombectomy system received 510(k) marketing ...
April 30, 2015 — Two global trials have found that the addition of the Solitaire device stent thrombectomy procedure to ...
March 12, 2015 — Pablo Uceda, M.D., vascular surgeon with DFW Vascular, Dallas, Texas, was successful in using a new ...
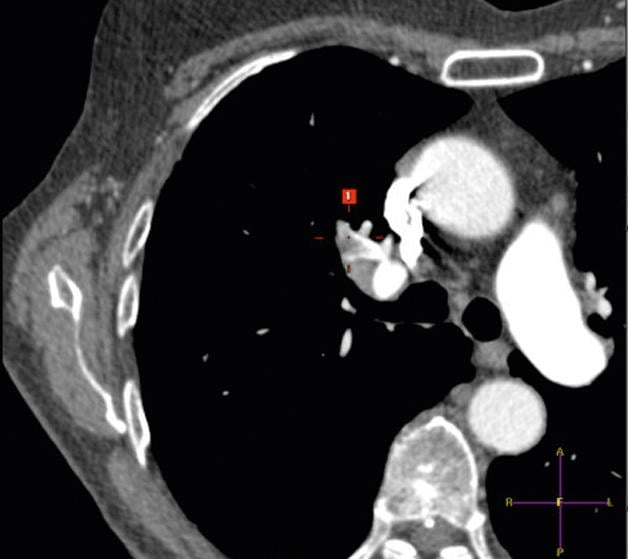
March 9, 2015 — NYU Langone Medical Center has announced the creation of a new multidisciplinary Venous Thromboembolic ...
February 12, 2015 — A manufacturing error caused the balloon inflation ports to be mislabeled on Covidien’s Trellis 6 ...

December 19, 2014 — In a landmark study evaluating the addition of stent thrombectomy clot removal to pharmaceutical ...
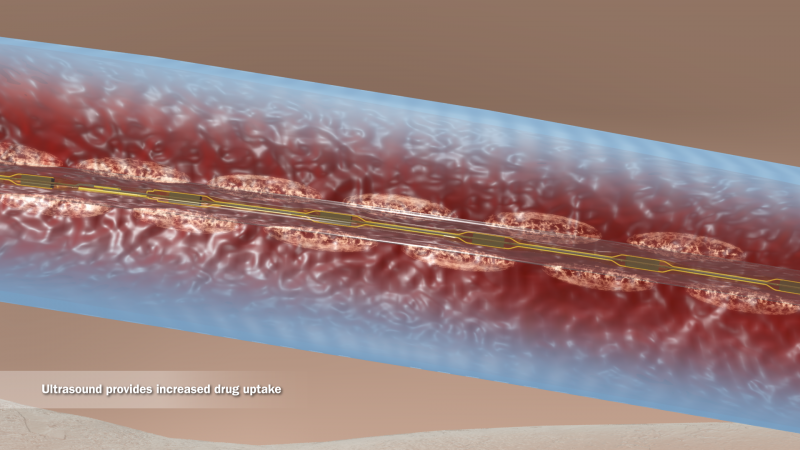
Acute intermediate-risk pulmonary emboli (PE) in normotensive patients with right ventricle dysfunction present the ...


 March 08, 2016
March 08, 2016