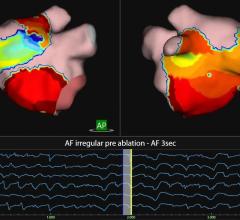
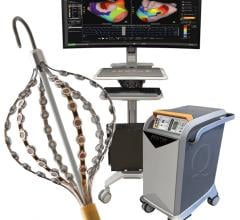
May 6, 2011 – Rhythmia Medical Inc. and Endosense have engaged in a joint development project to integrate the contact-force data provided by Endosense’s TactiCath force-sensing ablation catheter into Rhythmia’s advanced three-dimensional cardiac mapping, visualization and navigation system. The project’s objective is to build on earlier feasibility tests to develop an integrated solution that allows electrophysiologists to quickly, efficiently and effectively view catheter tip-to-tissue contact force on a high-resolution, 3-D electro-anatomical map.
The joint development project brings together two recognized innovators in the catheter ablation treatment of cardiac arrhythmias. Rhythmia Medical is pioneering new technology to allow for fast, simple and accurate 3-D mapping of electrical heart activity. Endosense pioneered the field of contact-force sensing with its TactiCath force-sensing ablation catheter and accompanying TactiSys system. By integrating their technologies, Rhythmia Medical and Endosense intend to provide electrophysiologists with an exceptional user experience to improve the patient outcomes of cardiac ablation procedures.
“By building Endosense’s contact-force sensing capability into Rhythmia Medical’s open platform advanced 3-D mapping, visualization and navigation system, we have the potential to deliver a new solution that will enhance the value of 3-D mapping with the greater insight and control of force-sensing into the EP lab,” said Leon Amariglio, co-chief executive officer of Rhythmia. “We are excited to be partnering with an industry leader and pioneer like Endosense to bring these significant benefits to electrophysiologists and their patients.”
Eric Le Royer, chief executive officer, Endosense, also commented on the development project. “We expect our joint effort with Rhythmia to yield a compelling new tool for electrophysiologists, as it will marry two very complementary, state-of-the-art technologies aimed at improving the efficacy, safety and ease of performing cardiac ablation procedures. I have witnessed the benefits of the speed, accuracy and ease of use of the Rhythmia system during the earlier pre-clinical feasibility tests we conducted together. We are happy to partner with this innovative company as part of our open platform strategy to integrate value-added contact-force sensing capability into advanced 3-D mapping systems.”
Endosense is also executing an open platform strategy to integrate its technology into a range of three-dimensional cardiac mapping systems. Biotronik is the exclusive distributor of the TactiCath in Europe, Latin America, Canada, Africa and the Middle East. The system is not yet cleared for use in the United States.
For more information: www.endosense.com, www.rhythmia.com.


 September 05, 2025
September 05, 2025