June 22, 2009 – Biosense Webster Inc. is showcasing the CARTO 3 System, an advanced three-dimensional imaging technology for use by electrophysiologists in treating cardiac arrhythmias this week at EUROPACE, the official Congress of the European Heart Rhythm Association.
The CARTO 3 System offers three unique features: Advanced Catheter Location (ACL) Technology, Fast Anatomical Mapping (FAM), and a streamlined workflow feature set referred to as Connection of Choice. The company said these three features work in tandem to enhance a physician’s ability to treat an array of simple and complex cardiac arrhythmias.
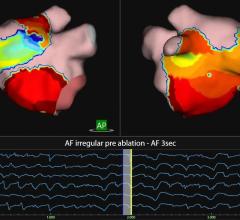
ACL allows for accurate visualization so physicians can clearly pinpoint all catheters in the anatomy with precision and clarity. It can visualize up to five catheters simultaneously with clear distinction of all electrodes. FAM quickly and accurately creates an anatomical map as a catheter is moved throughout the cardiac chamber similar to the way that a global positioning system can immediately track where a car may be. Images are high-resolution and similar to the clarity seen in CT-like maps, the company said.
Connection of Choice is enabled by a brand new CARTO hardware configuration featuring a central connection point for all catheters and equipment while preserving the signal quality of intracardiac electrograms. Catheter connections have been re-designed for “plug-and-play” functionality and automatic catheter recognition. All of these enhancements have been developed to streamline and simplify workflow in the EP lab.
“The CARTO 3 System is an exciting technology that represents a major step forward for electrophysiologists,” said professor Gerhard Hindricks, M.D., University Leipzig - Heart Center, department of cardiology, in Leipzig, Germany. “I was especially impressed with the Fast Anatomical Mapping feature. I was able to create a map quickly and the accuracy was excellent. In addition, I was pleased with how the system merged with our CT scan. Overall, the evaluation was a great experience for the whole lab team who enjoyed the new quick system set up.”
“After performing 10 procedures using the CARTO 3 technology, I believe this system will increase the use of ablation to treat Afib,” said Dr. Carlo Pappone, director of the department of arrhythmology at San Raffaele University Hospital in Milan, Italy, another of the EPs participating in the external evaluation for CARTO 3.
The CARTO 3 System will be released throughout the European Union, Australia and Canada in the third quarter of 2009. The CARTO 3 System is currently pending U.S. FDA 510(k) review and clearance.
Drs. Hindricks and Pappone are compensated for their time as consultants to Biosense Webster.
For more information: www.biosensewebster.com


 September 05, 2025
September 05, 2025