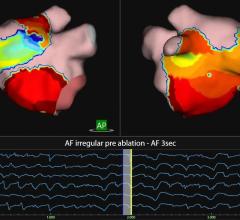
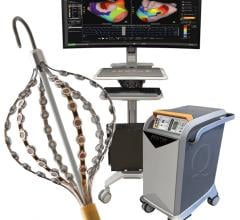
May 13, 2013 — Boston Scientific has received CE Mark approval for the Rhythmia Mapping System, a 3-D mapping and navigation solution for use in cardiac catheter ablations and other electrophysiology (EP) procedures to treat a variety of conditions in which the heart beats abnormally. Some of those conditions include atrial flutter, atrial fibrillation and ventricular tachycardia. Boston Scientific is offering the Rhythmia Mapping System with the company's 64 electrode IntellaMap Orion Mapping Catheter, which has also received CE Mark approval. The combination, part of the 2012 Rhythmia Medical acquisition, is designed to provide electrophysiologists with accurate, high-resolution electro-anatomical maps.
"In a pair of independent clinical studies, we repeatedly showed that the Rhythmia Mapping System can rapidly generate and display high density electro-anatomical maps that allowed clinicians to effectively diagnose and treat even the most complex patients," said Hiroshi Nakagawa, M.D., Ph.D., professor of medicine, director, Clinical Catheter Ablation Program, University of Oklahoma Health Sciences Center, and one of the principle investigators in the studies. "The IntellaMap Orion Catheter has a sophisticated 64 electrode design and unique deployable basket that provided a high degree of maneuverability."
EP mapping and navigation systems have become a standard tool for physicians performing catheter ablations, and current systems demand tradeoffs between accuracy and speed. A more accurate, high-resolution image may improve a physician's ability to select the appropriate site to ablate, improving procedural efficacy. Similarly, increasing the speed at which a mapping system can provide a high-resolution map may significantly reduce procedure time. The Rhythmia Mapping System is designed to increase speed and improve density of mapping compared to existing systems, potentially offering significant benefit to patients, physicians and health care systems.
In the United States, the Rhythmia Mapping System is an investigational device and not available for sale.
For more information: www.bostonscientific.com


 September 05, 2025
September 05, 2025