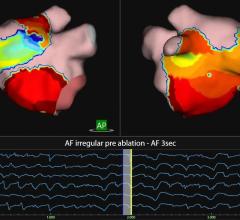
January 19, 2011 – Siemens Healthcare is partnering with Endosense for the integration of the contact-force data provided by Endosense’s TactiCath force-sensing ablation catheter with Siemens’ electrophysiology (EP) solutions. The goal of the effort is to develop an all-in-one software application that allows electrophysiologists to visualize catheter-tip-to-tissue contact force within a three-dimensional anatomic heart model during catheter ablation procedures.
“The joint development project between Endosense and Siemens represents a very promising area of future advancement in the field of catheter ablation, as it will offer electrophysiologists a new platform for incorporating contact force sensing into clinical practice,” said Eric Le Royer, Endosense CEO. “An integrated, all-in-one system will deliver greater functionality and efficiency to EP labs, thereby moving Endosense another step toward our goal of improving and expanding catheter ablation for the treatment of cardiac arrhythmias.”
Endosense pioneered the field of contact force sensing in catheter ablation with its TactiCath force-sensing ablation catheter and accompanying TactiSys system. Launched in Europe in April 2010, the second generation of the novel device is now used by electrophysiologists across Europe for the treatment of atrial fibrillation (AF) and supraventricular tachycardia (SVT). In the United States the TactiCath is an investigational device in the recently launched TOCCASTAR investigational device exemption (IDE) study.
“We believe optimization of catheter-tip-to-tissue contact force bears huge potential to improve effectiveness and efficiency of electrophysiology ablation procedures. However, it is important to visualize this new parameter in relation to the 3-D anatomy of the heart and, if available, planning data,” said Martin Ostermeier, director, innovations, of the interventional X-ray business unit of Siemens Healthcare. “The goal of our joint project is to demonstrate the clinical benefits of Endosense’s TactiCath technology integrated into the advanced 3-D imaging capabilities, the syngo DynaCT cardiac of our Artis zee family of cath lab angiography systems for EP procedures.”
For more information, visit www.endosense.com, www.siemens.com/healthcare


 September 05, 2025
September 05, 2025