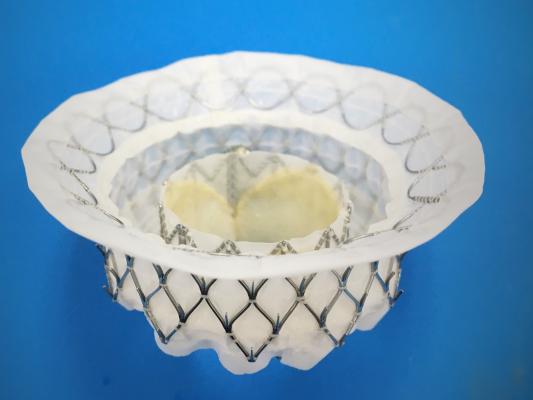
September 11, 2020 – Medtronic announced U.S. Food and Drug Administration (FDA) approval of an early feasibility study (EFS) of the Intrepid Transcatheter Tricuspid Valve Replacement (TTVR) system. The study begins on the heels of a recent Breakthrough Device Designation issued by the FDA for the Intrepid TTVR System. The Intrepid TTVR system is an investigational device worldwide.
The study will include patients with severe, symptomatic tricuspid regurgitation, a disease in which the diseased, damaged or malfunctioning tricuspid valve allows blood to flow back into the heart's upper right chamber causing eventual heart failure or death.
“We’re beginning a new journey that we believe will open the door for the potential future treatment of patients with tricuspid valve regurgitation, who constitute a significant, patient population suffering from heart valve disease today,” said Azeem Latib, M.D., section head of interventional cardiology and medical director of structural heart interventions at Montefiore Medical Center in New York City and co-principal investigator in the study. “There has been much progress regarding transcatheter replacement of diseased aortic valves, but whether we can replace the tricuspid valve without open heart surgery represents a new frontier in cardiology.”
Representing a large, unmet clinical need, tricuspid regurgitation affects more than 2 million patients in the United States. It is a highly under-treated disease due to the morbidity and mortality associated with surgical intervention.
Medtronic recently received Breakthrough Device Designation by the FDA for the Intrepid TTVR system. The FDA Breakthrough Device Program is intended to help patients receive more timely access to certain technologies that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions.
“The clinical experience generated during this initial study phase will be critical for the future of the therapy, as many of these patients are not good candidates for traditional surgical tricuspid valve interventions due to their poor right heart functions and are higher risk due to co-morbidities,” said Vinayak (Vinnie) Bapat, M.D., chief of cardiothoracic surgery at the Minneapolis Heart Institute and co-principal investigator in the study. “We are optimistic that these early learnings will help fuel additional clinical research and device innovation around this treatable disease.”
The Intrepid transcatheter valve is the same valve being evaluated for the treatment of symptomatic mitral valve regurgitation in the transfemoral mitral early feasibility study. The device is implanted using a transfemoral delivery catheter, which assists physicians in delivering and placing the valve through a catheter inserted in the femoral vein.
For more information: www.medtronic.com
Related Content on the Intrepid Transcatheter Valve:
Developments in Transcatheter Mitral Valve Replacement
8 Cardiovascular Technologies to Watch in 2020
Recent Advances in Transcatheter Valve Technology
University Hospitals Performs Ohio's First Transcatheter Mitral Valve Replacement
Medtronic Unveils Strong Early Outcomes for Intrepid Transcatheter Mitral Valve Replacement System
First Patient Treated in Pivotal Trial for Medtronic TMVR Valve


 January 05, 2026
January 05, 2026 









