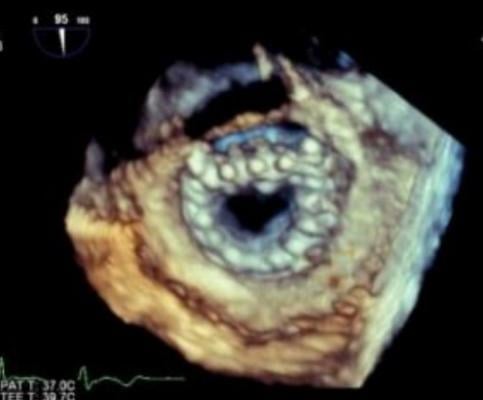
A 3-D echo view of a Neovasc Tiara transcatheter mitral valve. This valve is currently in clinical trials and is ahead of most of the TMVR devices in development.
May 4, 2018 — Transcatheter valve technology has been advancing very quickly and the links to aggregated content from the past year below offer a concise update. Transcatheter aortic valve replacement (TAVR) use has greatly expanded over the past several years. The Boston Scientific Lotus TAVR valve is now positioned to most likely become the third TAVR device available on the U.S. market in the next 18 months. Several transcatheter mitral valve replacement (TMVR) and mitral valve repair technologies are now in clinical trials in the U.S. and overseas. Transcatheter tricuspid valve devices are also now in trials, and a transcatheter tricuspid annual repair device was just cleared for use in Europe this week.
Edwards Granted CE Mark For First Transcatheter Tricuspid Therapy
VIDEO: Clinical Outcomes With the Lotus TAVR Valve - interview with Ted Feldman, M.D.
Hospital Consolidation May Increase Access to TAVR, New Cardiac Technologies
VIDEO: Transcatheter Mitral Valve Implantation in Practice and Technologies in Development - Interview with Adam Greenbaum, M.D.
How to Perform Transcaval TAVR Access
VIDEO: How Consolidation Into Larger Health Systems Can Improve Access to TAVR
Boston Scientific Prevails in European Patent Dispute With Edwards Lifesciences
TAVR Can Cause Silent Cerebral Microbleeds, Neurological Impairment
University Hospitals Performs Ohio's First Transcatheter Mitral Valve Replacement
VIDEO: Outcomes Following Urgent TAVR - Results from the TVT Registry - interview with Sammy Elmariah, M.D
Boston Scientific Projects 2019 Launch for Lotus Edge Aortic Valve System
Edwards Completes Enrollment in PARTNER 3 Low-Risk CT Sub-Study
VIDEO: TAVR For Asymptomatic Severe Aortic Stenosis - Interview with Philippe Genereux, M.D.
CoreValve TAVR System Shows Strong Long-Term Performance in Clinical Trials
VIDEO: TAVR for Degenerated Surgical Valves — Valve-in-Valve TAVR Procedures - interview with Sammy Elmariah, M.D
Sentinel Cerebral Protection System Reaches 50-Center Adoption Milestone in U.S.
Edwards Lifesciences Self Expanding TAVR Valve Gains European Approval
ACC Experts Share What Trends are Ahead in Cardiovascular Medicine in 2018?
Boston Scientific Enters Investment and Acquisition Option Agreement With Millipede
Edwards LifeSciences Recalls Sapien 3 Certitude Delivery System
Enrollment Completed in U.S. IDE Trial for Manta Large Bore Vascular Closure Device
Edwards Acquires Harpoon Medical
VIDEO: Applications in Cardiology for 3-D Printing and Computer Aided Design — interview with Dee Dee Wang, M.D.
VIDEO: Cath Lab of the Future Cardiovascular Technologies to Watch — interview with Juan Granada, M.D.
VIDEO: MAVERIC 6-month Outcomes for the Arto Mitral Valve Reshaping System
Harpoon Device May Improve Quality, Frequency of Mitral Repair
VIDEO: Update of Mitral Valve Repair and Replacement Technologies - Interview with Ted Feldman, M.D.
Positive, Sustained Improvement at One Year in SCOUT I Transcatheter Tricuspid Repair Trial
Medtronic Unveils Strong Early Outcomes for Intrepid Transcatheter Mitral Valve Replacement System
TAVR Cost-Effective Compared With SAVR in Intermediate-Risk Aortic Stenosis Patients
Sapien 3 Valve Demonstrates Significant Cost Savings Over Surgery In Intermediate-Risk Patients
Second Micro Interventional Devices Annuloplasty System Successfully Implanted
Materialise Announces Start of Pre-Market Phase for Mitral Valve Planning
VIDEO: Transcaval Access in TAVR Procedures - Interview with Adam Greenbaum, M.D.
Claret Medical Closes $14.5 Million in Series C Financing for Sentinel Cerebral Protection System
VIDEO: Conscious Sedation for TAVR Procedures — interview with Mario Goessl, M.D.
Sentinel Cerebral Protection System Significantly Reduces Stroke and Mortality in TAVR
Minneapolis Heart Institute Foundation Enrolls First Patient in TRILUMINATE Tricuspid Repair Trial
VIDEO: Mitral and Tricuspid Transcatheter Repair Technologies — interview with Azeem Latib, M.D.
Abbott Initiates First Clinical Trial of Clip-Based Tricuspid Repair System
Transcatheter Mitral Valve Repair Start-up CoreMedic Gets Financing
FDA Expands CoreValve TAVR Valve Use for Intermediate Risk Patients
Mitralign Announces First European Subject Enrolled in SCOUT II Study of Trialign System
BioTrace Medical's Tempo Lead Shows Stability and Safety in Real-World Performance
VIDEO: Transcatheter Tricuspid Valve Repair and Replacement Technologies — interview with Rebecca Hahn, M.D.
FDA Clears First TAVR Embolic Protection System
FDA Clears Aortic, Mitral Valve-In-Valve Procedures for Sapien 3 TAVR Valve
Lotus TAVR System Shows Superior Efficacy to CoreValve in Global REPRISE III Trial
Analysis Looks at Role Type of Valve Plays in Patient Outcomes Post-TAVR
Boston Scientific to Acquire TAVR Maker Symetis
VIDEO: TAVR Stands Equal to Surgical Valve Replacement — interview with Michael Reardon, M.D.
Transcatheter Annuloplasty For Repair Versus Replacement in Functional Mitral Regurgitation


 November 14, 2025
November 14, 2025 









