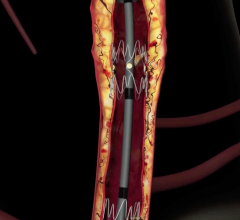
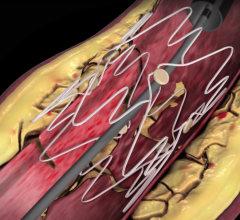
September 7, 2007 - Gary Ansel, M.D., Riverside Methodist Hospital, successfully implanted the GORE VIABAHN Endoprosthesis with Heparin Bioactive Surface, a stent graft designed to open blockages in the superficial femoral artery in the thigh, which is used to treat a common form of peripheral arterial disease (PAD).
The stent is designed to improve outcomes, speeds recovery and may minimize the risk of complications. The device, manufactured by W.L. Gore & Associates in Flagstaff, AZ, and FDA approved in August to relieve hardening of the thigh artery, uses the same material that is in rain suit for golf. The Gore-Tex fabric stops tissue from growing inside the tube and has the blood thinning medication Heparin as part of the device lining. The Gore-Tex keeps the Heparin anchored to its surface, allowing the medication to interact with the blood reportedly for years.
The stent placement is a less invasive alternative to bypass surgery that requires an incision or several incisions over the entire length of the thigh with a four- to six-week recovery. Ansel inserted the device in the patient’s leg in 20 minutes through a puncture the size of a ballpoint pen tip.
For more information: www.ohiohealth.com
News | September 09, 2007


 November 06, 2019
November 06, 2019