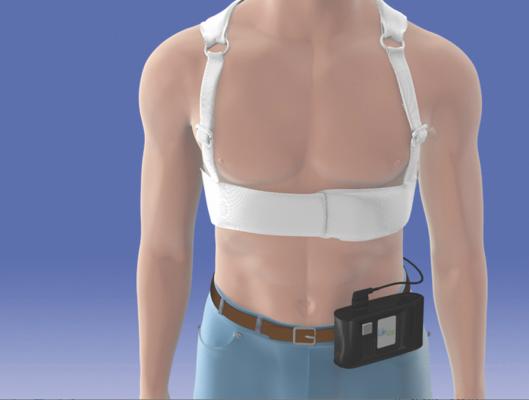
For patients at risk for sudden cardiac arrest (SCA) who are being evaluated for a permanent implantable cardioverter defibrillator (ICD), Zoll offers the LifeVest wearable defibrillator (WCD) as a temporary solution. It allows a physician more time to assess a patient’s long-term arrhythmic risk and make appropriate plans.
The LifeVest is lightweight and easy to wear under a patient’s clothing, allowing them to return to their daily activities while having the peace of mind that they are protected from SCA. The LifeVest continuously monitors the patient’s heart and, if a rapid life-threatening heart rhythm is detected, the device delivers a treatment shock to restore normal heart rhythm. This device continuously monitors the patient’s heart with dry, non-adhesive sensing electrodes to detect life-threatening abnormal heart rhythms. If a rapid life-threatening heart rhythm (VT or VF) is detected, the device alerts bystanders and delivers a treatment shock to restore normal heart rhythm. The entire event, from detecting a life-threatening arrhythmia to automatically delivering a treatment shock, usually occurs in less than a minute.
The LifeVest is used for a range of patient conditions or situations, including following a heart attack and before or after bypass surgery or stent placement, as well as for those with cardiomyopathy or congestive heart failure that places them at particular risk.
Zoll intregrates this device with the LifeVest Network online patient data management system. It allows clinicians to monitor the patient using downloaded data from a patient’s LifeVest. Clinicians can customize events that generate an alert, or to be notified for a number of events, such as detected arrhythmias, treatments, patient-recorded electrocardiograms (ECGs) and patient use.
Other LifeVest Related Content:
Sudden Cardiac Arrest Patient Walks to Ambulance Thanks to Wearable Defibrillator
VIDEO: Demonstration of the LifeVest Wearable Defibrillator System
Diving Deeper Into the Results of the VEST Trial Using Wearable Defibrillators
Wearable Cardioverter Defibrillators Prove Safe for Pediatric Patients
New Technology and Market Challenges Facing Implantable Cardioverter Defibrillators


 January 29, 2026
January 29, 2026 









