March 20, 2012
March 20, 2012 - MarBlue Medical announced the global product launch of its CE-marked drug-eluting balloon (DEB)…
March 20, 2012
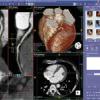
March 20, 2012 - GE Healthcare is demonstrating its new Centricity Cardio Enterprise solution during the American…
March 20, 2012
March 20, 2012 - DR Systems announced new contracts with four facilities for the company's Unity CVIS (Cardiovascular…
March 19, 2012
Jack Miller, 62, is probably the only politician in America who smiles when people call him “heartless.” Four months…
March 19, 2012
March 19, 2012 - Cameron Health Inc. announced today that the U.S. Food and Drug Administration (FDA) Circulatory…
March 19, 2012
In the past decade, there has been a major push toward minimally invasive cardiac procedures. In the cath lab,…
March 19, 2012
Management of coronary chronic total occlusion (CTO) is individualized depending on the severity of symptoms, ischemia…
March 19, 2012
Cardiology departments have hosted a variety of software solutions in the past to meet varying demands. A recent report…
March 19, 2012
Computer-aided detection (CAD) software constitutes a set of algorithms which, using a pattern-recognition technique,…
March 19, 2012
March 19, 2012 – Individuals suffering from chronic heart failure (CHF) can look forward to the appearance of many new…
March 19, 2012
Rates of sudden cardiac death due to heart rhythm abnormalities remain at high levels despite significant advances in…
March 19, 2012
March 19, 2012 - Biomedical Systems, a global provider of cardiac diagnostic services and products, has introduced the…
March 19, 2012
Transcatheter heart valves are viewed as the poster children devices representing the latest in cutting-edge cardiac…
March 19, 2012
March 16, 2012 -- Kensey Nash Corp. today announced that the company has entered into a $39 million settlement…
March 16, 2012
We are at an inflection point with many aspects of medicine, not the least of which concerns choosing the correct tool…
March 16, 2012
April 15, 2012, will mark the 10th anniversary of the approval of the first drug-eluting stent (DES). Ten years ago,…
March 16, 2012
Hospitals that have built multimillion-dollar, specialized cardiovascular hybrid operating rooms (ORs) are hoping to…
March 16, 2012
March 16, 2012 — The U.S. Food and Drug Administration (FDA) approved the premarket approval (PMA) application for the…
March 16, 2012
March 16, 2012 — Epocrates is releasing two new, free mobile device applications for cardiologists during the American…
March 16, 2012
March 16, 2012 - Stroke survivors who like art have a significantly higher quality of life than those who do not,…