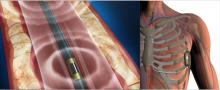
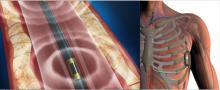
March 8, 2021 — University of Kansas Health System Cardiologist Ashley Simmons, M.D., knew there could and should be a better way to measure female heart function. She had seen countless women balk at the uncomfortable testing that requires patients to go bra-less.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now