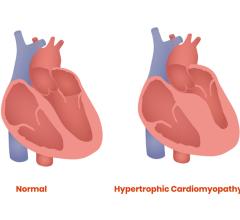
May 13, 2022 — Bristol Myers Squibb’s mavacamten will stand rather unchallenged in the hypertrophic cardiomyopathy (HCM) ...
Pharmaceuticals
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, INR testing, NOAC, OAC, IV administered drugs such as Heprin, and dual antiplatelet therapy.
May 5, 2022 — High-level results from the DELIVER Phase III trial showed AstraZeneca’s FARXIGA (dapagliflozin) reached a ...
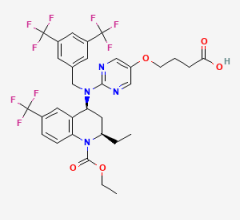
April 29, 2022 — Bristol Myers Squibb announced that the U.S. Food and Drug Administration (FDA) approved Camzyos ...
April 12, 2022 – A majority of patients with obstructive hypertrophic cardiomyopathy (HCM)—a condition that results in ...
April 8, 2022 – Patients taking renin-angiotensin-aldosterone system inhibitor (RAASi) medications for heart failure had ...
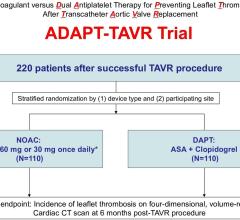
April 7, 2022 – Patients treated with the blood thinner edoxaban for six months after a heart valve replacement ...
April 7, 2022 – While there are effective therapies to reduce the risk of heart disease by lowering low-density ...
April 4, 2022 – Esperion presented two new analyses from its clinical development program of bempedoic acid (NEXLETOL) ...
March 31, 2022 – Windtree Therapeutics, a biotechnology company focused on advancing multiple late-stage interventions ...
March 22, 2022 – Fresenius Kabi, a global health care company that specializes in medicines and technologies for ...
March 4, 2022 – NewAmsterdam Pharma (NAP), a clinical-stage company focused on the research and development of ...
February 24, 2022 — The U.S. Food and Drug Administration approved Jardiance (empagliflozin) to reduce the risk of ...
January 5, 2022 — The Feinstein Institutes for Medical Research, the science arm of Northwell Health, recently received ...
January 4, 2022 — The Omicron variant, as of Dec. 25, made up 58.6% of new COVID-19 infections in the U.S., according to ...
January 4, 2022 — The U.S. Food and Drug Administration (FDA) has cleared Novartis' inclisiran (Leqvio), the first ...

 May 13, 2022
May 13, 2022