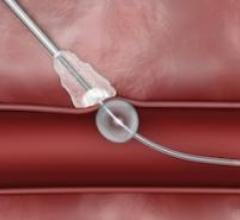
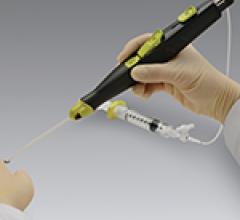
December 22, 2017 — Essential Medical announced the completion of enrollment in the U.S. pivotal investigational device ...
Vascular Closure Devices
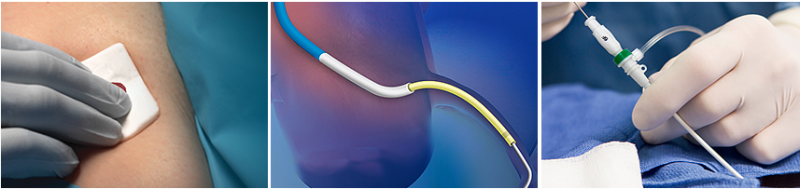
This channel includes news and new technology innovations for vascular closure devices used to rapidly seal and achive hemostatsis at vascular access sites in interventional cardiology procedures.
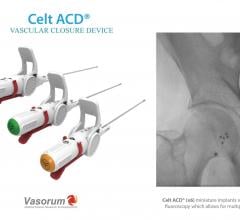
December 14, 2017 — Vasorum Ltd, the developer and manufacturer of the novel Celt ACD vascular closure device, has added ...
December 7, 2016 — Teleflex Inc. and Vascular Solutions Inc. announced that the companies have entered into a definitive ...
October 18, 2016 — Abbott and St. Jude Medical Inc. announced today an agreement to sell certain products from their ...
September 12, 2016 — Vivasure Medical announced last week that the company has completed a Series C financing of €16.2M ...
August 3, 2016 — The U.S. Food and Drug Administration (FDA) has granted market clearance to Rex Medical’s bioresorbable ...
January 18, 2016 — Vivasure Medical announced Conformité Européenne (CE) Mark approval of the world’s first fully ...
December 16, 2015 — Scientists at CBSET have presented preclinical evidence that the Manta Large Bore Vascular Closure ...
November 6, 2014 — Cardinal Health announced that its MynxGrip Vascular Closure Device recently received U.S. Food and ...
Doug Drachman, M.D., Mass General Hospital Institute of Heart, Vascular and Stroke Care, explains how to prevent and ...
September 17, 2014 — A new clinical trial found that vascular closure devices (VCDs) are non-inferior to manual ...
August 21, 2014 — Cardiva Medical Inc. said it closed the first two tranches of a $16.5 million Series 3 private equity ...
August 7, 2014 — Transluminal Technologies LLC, a Syracuse, NY-based medical device company, received CE mark approval ...
June 13, 2014 — Cardinal Health announced the 2 millionth shipment of the Mynx Vascular Closure Device (VCD). The Mynx ...
April 8, 2014 — Cardinal Health announced an agreement to acquire privately held AccessClosure Inc. for $320 million ...

 December 22, 2017
December 22, 2017