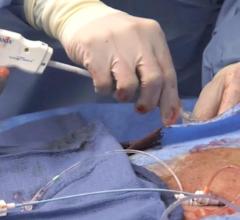
May 15, 2019 — Cordis, a Cardinal Health company, recently announced the full U.S. launch of its Radial 360 portfolio ...
Vascular Closure Devices
This channel includes news and new technology innovations for vascular closure devices used to rapidly seal and achive hemostatsis at vascular access sites in interventional cardiology procedures.
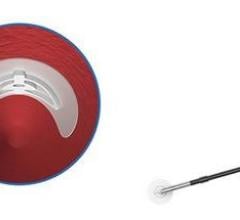
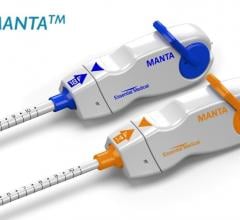
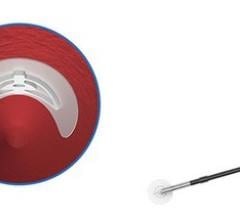
April 3, 2019 — Essential Medical Inc. received U.S. Food and Drug Administration (FDA) clearance for its large bore ...
December 20, 2018 — Cardiva Medical Inc. announced the company has received premarket approval (PMA) from the U.S. Food ...
November 21, 2018 – Vivasure Medical Ltd. recently announced the European launch of the PerQseal closure device for ...
November 12, 2018 — The Vascade MVP vascular closure system met its endpoints compared to manual compression in a ...
October 5, 2018 — Teleflex Inc. announced the acquisition of Essential Medical Inc. Based in Exton, Pa., Essential ...
Philippe Genereux, M.D., co-director of the structural heart program at the Gagnon Cardiovascular Institute at ...
May 10, 2018 — Cardiva Medical Inc. announced the completion of the AMBULATE pivotal trial, one of several clinical ...

The European interventional cardiology market is currently valued at nearly $1.4 billion. This is a mature market that ...
April 17, 2018 — The U.S. Food and Drug Administration (FDA) announced market approval for the Abbott Perclose ProGlide ...
April 10, 2018 – Cardiva Medical announced the company received U.S. Food and Drug Administration (FDA) approval for an ...
February 21, 2018 – Vascular closure device provider Cardiva Medical announced that the company has closed on $11 ...
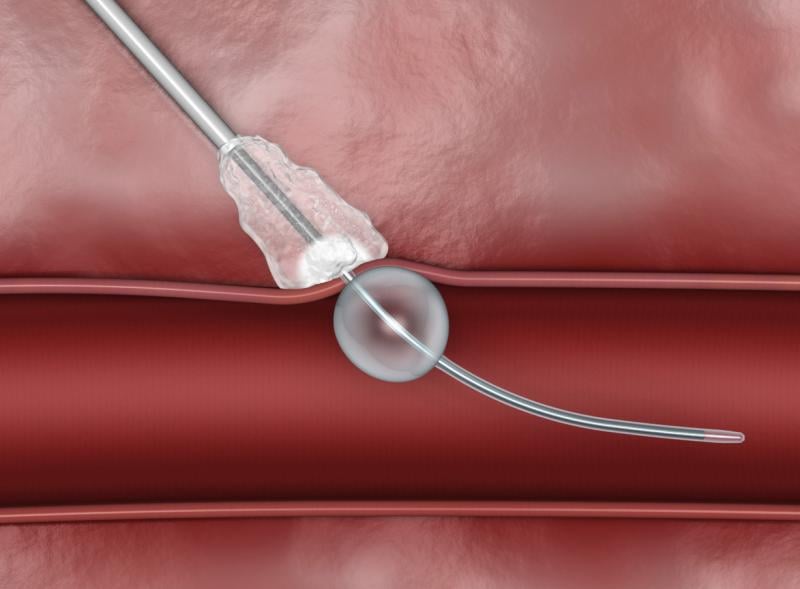
While manual compression remains the gold standard for hemostasis of catheterization vascular access site arteriotomies ...
December 29, 2017 — Vivasure Medical announced in October the successful enrollment of the first patient in the Frontier ...


 May 15, 2019
May 15, 2019