Office based labs (OBLs) and ambulatory surgery centers (ASCs) require a fresh…
Cardiovascular Systems Inc. recently announced U.S. Food and Drug Administration (FDA) premarket…
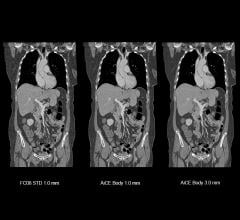
Canon Medical Systems USA Inc. has received U.S. Food and Drug Administration (FDA) 510(k)…
Abbott recently announced that its Architect Stat High Sensitivity Troponin-I blood test has…
Abiomed’s newest heart pump, the Impella 5.5 with SmartAssist, has received U.S. Food and Drug…
The U.S. Food and Drug Administration (FDA) has cleared three modules of AI-Rad Companion Chest…
Medtronic announced U.S. Food and Drug Administration (FDA) approval and U.S. launch of the…
The U.S. Food and Drug Administration (FDA) has cleared the Artis icono, a high-precision family…
Rex Medical L.P. has received 510(k) clearance from the U.S. Food and Drug Administration (FDA)…
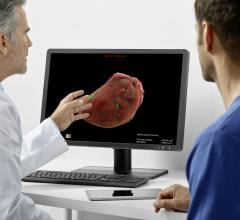
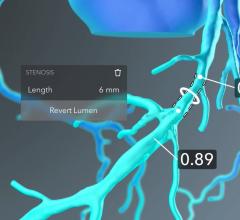
HeartFlow Inc. has obtained clearance from the U.S. Food and Drug Administration (FDA) for the…
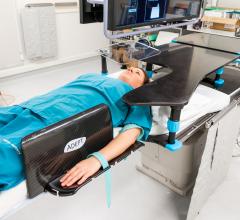
New Zealand-based Adept Medical announced the launch of the Antegrade IR Platform. Clinically…
August 23, 2019 — Cook Medical recently released the second generation of the 2.6 Fr CXI…
The U.S. Food and Drug Administration (FDA) approved the Barostim Neo System for the improvement…
Procyrion Inc. secured Breakthrough Device designation by the U.S. Food and Drug Administration…
Penumbra announced U.S. commercial availability of the Penumbra System’s most advanced…
Abbott has received U.S. Food and Drug Administration (FDA) approval for the most advanced…
July 15, 2019 — The U.S.
Philips recently announced new advanced automation capabilities on its Epiq CVx and Epiq CVxi…
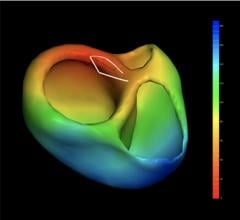
Catheter Precision Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared…
Siemens Healthineers and Mentice AB announced the collaboration to fully integrate Mentice’s…


 October 28, 2019
October 28, 2019