Mercom Capital Group llc, a global communications and research firm, released its report on funding and mergers and acquisitions (M&A) activity for the Healthcare Information Technology (IT)/Digital Health sector for the second quarter of 2015. The comprehensive report covers deals of all sizes across the globe.
Sorin S.p.A. and Cyberonics Inc. unveiled LivaNova as the name of their combined company, effective at the close of their proposed merger.
The Society for Heart Attack Prevention and Eradication (SHAPE) submitted a letter to the U.S. Preventive Services Task Force (USPSTF) strongly supporting the use of coronary artery calcium to assess an individual's risk of heart attack.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

C.R. Bard Inc. announced the publication of results from the LEVANT 2 study in the June 24, 2015, online issue of The New England Journal of Medicine. Results from LEVANT 2 demonstrated superior primary patency for the Lutonix 035 drug coated balloon percutaneous transluminal angioplasty (PTA) catheter over standard PTA, as well as safety consistent with standard PTA balloons. The Lutonix 035 DCB is an angioplasty balloon coated with a therapeutic dose of the drug paclitaxel, and also utilizes standard mechanical dilatation of the vessel to restore blood flow for patients with peripheral arterial disease (PAD) in the femoropopliteal arteries.
Masimo announced the CE Mark and full market release of MightySat Rx fingertip pulse oximeter for clinical use in CE countries. The device uses Masimo’s SET Measure-Through Motion and Low Perfusion pulse oximetry.
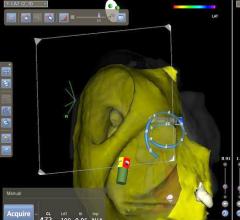
Biosense Webster Inc. announced the launch of the Confidense Module, an innovative technology that streamlines the creation of real-time 3-D maps of a patient’s cardiac structures during catheter ablation procedures. Utilizing an advanced, proprietary algorithm, the module enhances the process for validating information, or “points,†acquired during multi-electrode mapping, enabling electrophysiologists to focus their time and expertise on the most relevant data during the mapping process.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

A new document from the American Society of Echocardiography (ASE) provides detailed guidance for clinicians on how to use echocardiography to see the full picture of the condition of patients with hypertension. The paper, Recommendations on the Use of Echocardiography in Adult Hypertension: A Report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE) is a joint project between ASE and the European Association of Cardiovascular Imaging (EACVI) and will appear in the July issue of the Journal of the American Society of Echocardiography (JASE).
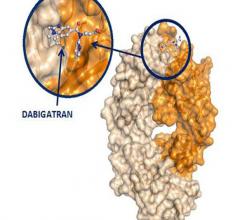
Results from an interim analysis of the Phase III RE-VERSE AD patient study demonstrate that 5 g of idarucizumab immediately reversed the anticoagulant effect of dabigatran (Pradaxa) in patients requiring urgent anticoagulant reversal. No safety concerns relating to idarucizumab were identified. The results have been simultaneously published in the New England Journal of Medicine (NEJM) and presented at the International Society of Thrombosis and Haemostasis 2015 Congress in Toronto, Canada.
Safety accidents drive up costs, damage reputations and lower patient satisfaction scores. For instance, if a patient falls off a system's table after being sedated and is badly injured, it can result in significant repercussions for the provider. This type of scenario is rare, but still causes concern for health systems nationwide.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Here are a few observations on cardiovascular ultrasound advances I made while attending the 2015 American Society of Echocardiography (ASE) annual meeting in June:
DC Devices Inc. announced that it has completed enrollment and implants in the REDUCE LAP-HF trial, an open label, multi-center, single-arm study of the InterAtrial Shunt Device (IASD). The IASD is a transcatheter device for the treatment of diastolic heart failure (DHF), which is also known as heart failure with preserved ejection fraction (HFpEF).
Medraysintell released its updated World Market Report and Directory on Nuclear Medicine, Edition 2015, in late June, providing a description and analysis of the latest developments in nuclear medicine. The 920-page document covers 335 radiopharmaceuticals and radionuclides and 160 companies and institutions active in nuclear medicine.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
University of Southampton scientists have discovered a link between coronary heart disease and osteoporosis, suggesting both conditions could have similar causes.

Boston Scientific has initiated a worldwide study to evaluate the rate and causes of shocks for patients implanted with the Emblem subcutaneous implantable defibrillator (S-ICD). The device is indicated for primary prevention of sudden cardiac death in the setting of severely reduced cardiac function (left ventricular ejection fraction </= 35 percent).
Medtronic plc announced it has acquired the assets of Aptus Endosystems Inc., a privately held medical device company focused on developing advanced technology for endovascular aneurysm repair (EVAR) and thoracic endovascular aneurysm repair (TEVAR). Medtronic completed its acquisition of the assets of Aptus Endosystems in a transaction valued at approximately $110 million. Additional terms of the acquisition were not disclosed.


 July 09, 2015
July 09, 2015