November 5, 2021 — The U.S. Food and Drug Administration (FDA) authorized the emergency use of the Pfizer-BioNTech COVID ...

November 4, 2021 — The Centers for Medicare and Medicaid Services (CMS) today issued an emergency regulation now ...
November 2, 2021 — Cardionomic Inc. announce initial U.S. enrollment in its global Cardiac Pulmonary Nerve Stimulation ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
November 2, 2021 — Datascope/Getinge/Maquet is recalling its Cardiosave Hybrid/Rescue Intra-Aortic Balloon Pumps (IABP) ...
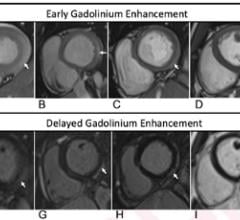
November 1, 2021 — According to ARRS’ American Journal of Roentgenology (AJR), radiologists need to be cognizant of the ...

November 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
November 1, 2021 — Health AI company Vagus.co, based in Cambridge, U.K., has launched a 30-second breathing test for ...

October 29, 2021 — A new guideline for the evaluation and diagnosis of chest pain was released this week that provides ...
With 1.2 to 1.4 million new electrophysiology (EP) devices being prescribed to patients around the world each year ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
October 28, 2021 – The first patient has been treated in a new trial using stem cell therapy to treat chronic myocardial ...
October 28, 2021 — CathVision, a medical technology company developing electrophysiology (EP) solutions in EP recording ...
Accurate reporting is essential to producing the reliable analytics needed to meet lab accreditation standards. This is ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
October 27, 2021 — Impulse Dynamics announced the U.S. Food and Drug Administration approved a modification of labeling ...
October 27, 2021 — EBR Systems Inc., developer of the world’s first wireless cardiac pacing system for heart failure ...
October 27, 2021 — Teleflex Inc. announced the completion of patient enrollment in a clinical study evaluating the ...

 November 05, 2021
November 05, 2021
![Figures from an initial study on the Cardionomic CPNS technology in a poster presentation at the Heart Rhythm Society (HRS) 2021 meeting.[1] Starting at top left, an angiographic view of the atrial transseptal access for a left ventricular septal ablation. Procedural intra-cardiac echo (ICE) showing ablation catheter positioning. A 3D electro-anatomic map of the LV septum. The final graphs show baseline and post-procedure LVOT pressure readings demonstrating a decreased gradient.](/sites/default/files/styles/content_feed_medium/public/Cardionomic_Cardiac_Pulmonary_Nerve_Stimulation_CPNS_for_HF.jpg?itok=ecrU9hmQ)