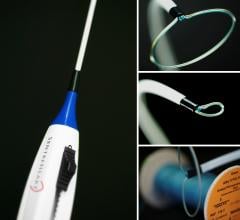
Boston Scientific announced in late February that the Eluvia Drug-Eluting Vascular Stent System received CE Mark, and is commencing commercialization immediately in the European Union and other countries where CE Mark is recognized.
Clarius Mobile Health is showcasing a new handheld ultrasound scanner with a mobile application for iOS and Android smart devices at the American Institute of Ultrasound in Medicine Conference in New York, March 18-21.
A recent study is bringing new hope to families of children who struggle with a common form of leukemia and thus must confront an elevated risk for long-term heart ailments caused by chemotherapy.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The Valley Hospital in Ridgewood, N.J., is one of 15 U.S. sites currently enrolling patients in a research study evaluating a potential new treatment for patients with symptomatic persistent and long standing persistent atrial fibrillation (AFib).
Bioengineers and physicians at the University of California, San Diego have developed a potential new therapy for critical limb ischemia, a condition that causes extremely poor circulation in the limbs. The new therapy could prevent or limit amputations for a condition that affects more than 27 million people and is a manifestation of advanced peripheral arterial disease.
A team of researchers led by the University of Missouri School of Medicine has developed a new, real-time method of imaging molecular events after ischemic strokes ― a finding that may lead to improved care for patients.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

An independent panel of experts convened by the U.S. Food and Drug Administration (FDA) voted 9 to 0, with one abstention, that the benefits of Abbott's Absorb fully bioresorbable drug eluting coronary stent outweigh the risks. The device will now go to the FDA for a final decision on market approval, likely later this year.
A new study from Juniper Research has estimated that the adoption of health monitoring devices will nearly treble by 2020, exceeding 70 million worldwide, up from an estimated 26 million this year.
People who reach their 80s without cardiovascular disease are more likely to suffer from the effects of dementia than a heart attack or stroke, according to a study in the Journal of the American College of Cardiology (JACC). In a small group of participants, an association was also found between zero or low levels of artery-clogging calcium deposits and a low risk of dementia and cardiovascular events, suggesting that the cardiovascular risk factors that lead to coronary heart disease could also affect the brain.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Dictum Health Inc. unveiled its Virtual Exam Room (VER), the centerpiece of its cloud-based, cybersecure and HIPAA-compliant telehealth system.
The Centers for Medicare and Medicaid Services (CMS) and the Office of the National Coordinator for Health Information Technology (ONC HIT) announced a new initiative to improve interoperability between Medicaid providers. The initiative, announced by CMS Acting Administrator Andy Slavitt and ONC Karen DeSalvo, is designed to improve patient care by distributing information and best practices to provide a better experience of care for individuals in the health system.
Biotronik announced that its BioMonitor 2 heart monitor is now available for patients in the United Kingdom. The insertable device allows highly accurate and reliable continuous detection of cardiac electrical events.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
As the healthcare industry evolves from a volume- to a value-based payment system, clinical information technology (IT) executives are expected to play an increasingly significant role in ensuring health IT is appropriately leveraged to positively impact care outcomes. Evidence from the 2016 HIMSS Leadership Survey, released at the Healthcare Information and Management Systems Society (HIMSS) annual conference and exhibition in Las Vegas, clearly support these expectations.
The 2016 HIMSS Connected Health Survey paints an optimistic picture surrounding the emerging trend of connectivity within the healthcare ecosystem. With more than 50 percent of respondents indicating their hospital currently uses three or more connected health technologies, the high adoption rates (and other supportive statistics in the report) underscore the growing importance these technologies play in the hospital setting.
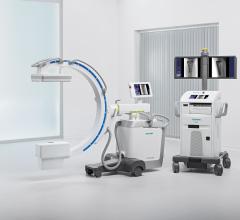
Siemens Healthcare announced the U.S. Food and Drug Administration (FDA) has cleared three brand-new system models in the company’s Cios family of mobile C-arms – Cios Fusion, Cios Connect and Cios Select. These new systems join a newly upgraded version of Siemens’ established, ultra-premium Cios Alpha mobile C-arm.


 March 17, 2016
March 17, 2016