May 2, 2016 — Starting at the Society for Cardiovascular Angiography and Interventions (SCAI) 2016 annual meeting May 4 ...
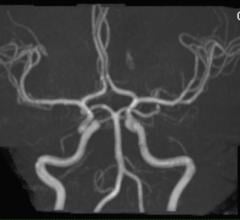
Bayer announced that the U.S. Food and Drug Administration (FDA) has approved Gadavist (gadobutrol) injection for use with magnetic resonance angiography (MRA) to evaluate known or suspected supra-aortic or renal artery disease.
April 29, 2016 — AtriCure Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the AtriClip PRO2 ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Using new high-resolution microscopy, researchers have found that molecular struts called microtubules (MT) interact with the heart’s contractile machinery to provide mechanical resistance for the beating of the heart.
Konica Minolta introduced the latest version of the Sonimage HS1 compact ultrasound system, enabling improved image quality, streamlined workflow and new cardiac functionality for the point-of-care ultrasound market.
Boston Scientific has received U.S. Food and Drug Administration (FDA) approval for a suite of products deemed safe for use in a magnetic resonance imaging (MRI) environment.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
April 28, 2016 — Breast cancer patients undergoing treatment with trastuzumab-containing regimens should be monitored ...

April 28, 2016 — Abbott and St. Jude Medical Inc. announced a definitive agreement for Abbott to acquire St. Jude ...
The Department of Health and Human Services (HHS) issued a proposal to align and modernize how Medicare payments are tied to the cost and quality of patient care for hundreds of thousands of doctors and other clinicians.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 27, 2016 — Imaging Advantage LLC, platform provider of cloud-based radiology service, announced the launch of a ...
Veniti Inc. announced the first successful treatment with the Vici Verto Venous Stent System of a patient suffering from post-thrombotic syndrome (PTS) associated with venous outflow obstruction.
Mitralign Inc. announced its Mitralign Percutaneous Annuloplasty System (MPAS) has received CE mark approval from its notified body, the British Standards Institution (BSI), for the treatment of functional mitral regurgitation (FMR).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The results of a secondary analysis of the SCOT-HEART trial show that coronary computed tomography angiography (CCTA) leads to a more appropriate and effective selection of invasive coronary angiography for patients with suspected angina due to coronary heart disease.
Taking long naps or being excessively tired during the day is associated with a higher risk for developing metabolic syndrome, according to a study presented at the American College of Cardiology’s 65th Annual Scientific Session.
Auris Surgical Robotics Inc. and Hansen Medical Inc. announced that they have signed a definitive merger agreement under which Auris will acquire Hansen Medical for $4.00 per share in cash.


 May 02, 2016
May 02, 2016