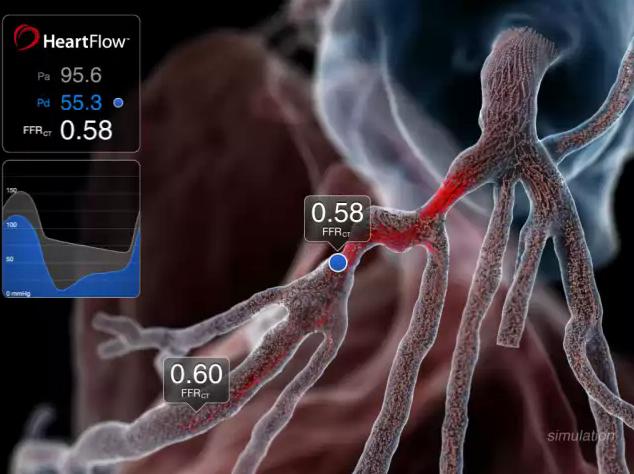
Results of the PLATFORM trial indicate fractional flow reserve computed tomography (FFR-CT) can obviate the need for invasive tests in up to 61 percent of patients with chest pain and suspected coronary artery disease. The results were presented at the European Society of Cardiology (ESC) Congress 2015.

Hospital-based healthcare providers today are under greater pressure than ever to administer cost- and time-efficient ...
An investigational material known as Bioabsorbable Cardiac Matrix (BCM), designed to prevent cardiac remodeling in heart attack patients, had no significant effect compared to a saline placebo, according to a new study.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
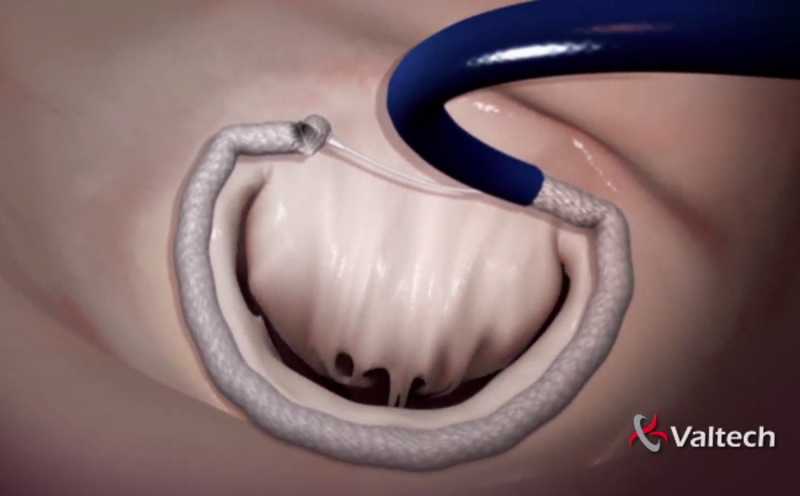
September 2, 2015 — HeartWare International Inc. announced it entered into a definitive agreement to acquire Valtech ...
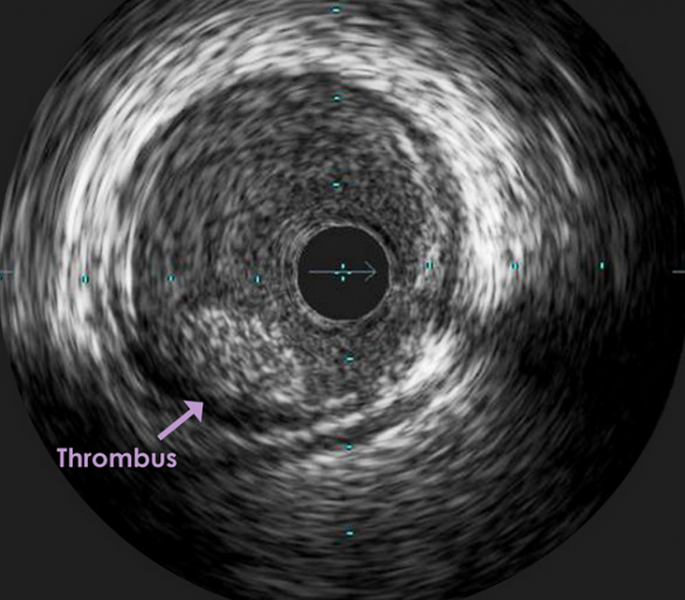
Retrieval of larger thrombi during intra-arterial treatment (IAT) is associated with improved neurological recovery after acute ischemic stroke, according to a sub-study of the MR CLEAN trial.

Findings presented at the European Society of Cardiology (ESC) Congress 2015 indicate that a small left ventricle with thick walls is the strongest predictor of morphologic remodeling in chronic ischemic heart disease patients.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Emergency department patients with chest pain suggestive of acute myocardial infarction (AMI) can be triaged more quickly and safely using a new rapid assay with refined cut-offs, German research suggests.
Automated alerts for excess fluid accumulation in the lungs did not improve outcomes for heart failure patients with implantable cardioverter defibrillators (ICDs), according to results of the OPTILINK HF trial.
The first-ever two-year outcomes from the Global Anticoagulant Registry in the Field - Atrial Fibrillation (GARFIELD-AF) were showcased at the European Society of Cardiology (ESC) Congress 2015 in London.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Based on its recent analysis of the cardiovascular image management market, Frost & Sullivan recognizes Pie Medical Imaging (PMI) with the 2015 European Frost & Sullivan Award for Technology Leadership.
September 1, 2015 — A bioresorbable drug-eluting coronary stent showed similar efficacy and safety results compared to a ...
September 1, 2015 — Extending treatment with the anticoagulant bivalirudin for at least four hours after completion of ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Medtronic plc announced first-of-its-kind findings from two independent studies that have identified a gene associated with life-threatening abnormal heart rhythms. The study results were presented in a hotline session at the 2015 European Society of Cardiology (ESC) Congress in London.
Cardiologists failed to identify more than half of basic and about 35 percent of advanced pre-recorded murmurs, according to research presented at the European Society of Cardiology Congress 2015. Skills did improve, however, after a 90-minute training session.
NorthStar Medical Radioisotopes LLC has received approval to begin routine production of molybdenum-99 (Mo-99) at the University of Missouri Research Reactor (MURR) facility in Columbia, Missouri.

 September 03, 2015
September 03, 2015