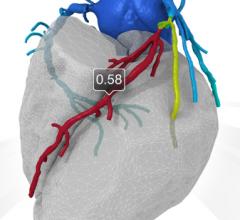
August 27, 2015 — The use of computational fluid dynamics (CFD) modeling has been used for years to better engineer ...
August 26, 2014 — The Cardiovascular Research Foundation (CRF) announced the late-breaking trials and first report ...
August 25, 2015 — Medtronic announced it entered a definitive agreement to acquire Twelve Inc., a start–up medical ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
August 25, 2015 — The American Society of Echocardiography Foundation’s (ASEF) humanitarian mission in Hanoi, Vietnam ...
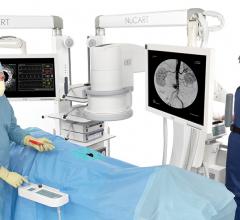
August 21, 2015 — CompView Medical announced the release of the NuCART, a turn-key, all-in-one mobile boom system with ...
August 21, 2015 — Loyola University Medical Center is the first and only hospital in Illinois to offer a new ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
BioSig Technologies announced it has signed a sponsored research agreement with the regents of the University of California at Los Angeles (UCLA) to conduct preclinical evaluation of BioSig's Pure EP System in a ventricular tachycardia (VT) model.
WomenHeart: The National Coalition for Women with Heart Disease announced that it will host the first National Policy & Science Summit on Women's Cardiovascular Health, Oct. 26-27, 2015 in Washington, D.C.
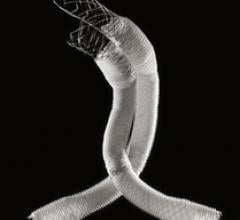
Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) approval for the Innova Vascular Self-Expanding Stent System, an advanced treatment option for patients with narrowing or blockages in the superficial femoral artery (SFA) or proximal popliteal artery (PPA).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Lombard Medical Inc. a medical device company focused on endovascular aneurysm repair (EVAR) of abdominal aortic aneurysms (AAAs), announced the acquisition of Silicon Valley-based Altura Medical.
Corindus Vascular Robotics Inc. and Unfors RaySafe Inc., a Fluke Biomedical Company, announced a distribution agreement allowing Corindus to offer the RaySafe i2 real-time radiation dose monitoring system in conjunction with the CorPath System.
Loyola University Medical Center is the first center in Illinois to implant the CoreValve Evolut R percutaneous aortic heart valve that does not require open heart surgery.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Women and black patients lost more years of their expected life after a heart attack when compared to white men, according to a study published in the Journal of the American College of Cardiology.
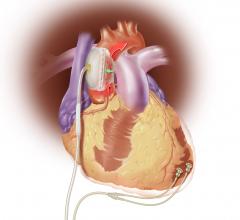
Sunshine Heart Inc. announced an update in late July on its COUNTER HF U.S. pivotal study for the C-Pulse Heart Assist System. COUNTER HF is a prospective, randomized, multi-center, controlled study evaluating the safety and efficacy of the C-Pulse system for the treatment of New York Heart Association (NYHA) Class III and ambulatory Class IV heart failure. The study was temporarily paused this past March after the company notified the U.S. Food and Drug Administration (FDA) of four deaths in the treatment arm of the study. The deaths were adjudicated as not device- or therapy-related, and as previously announced on May 26th, the FDA approved resumption of patient enrollment in the study.
Merit Medical Systems Inc. announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) expanding indication for QuadraSphere Microspheres to include the embolization of hepatoma.

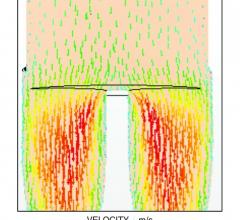
 August 27, 2015
August 27, 2015