Qinghui Chen, assistant professor in Michigan Tech’s kinesiology and integrative physiology department, wants to get to the bottom of two cardiovascular diseases: hypertension and congestive heart failure.
The U.S. Food and Drug Administration (FDA) approved Boston Scientific’s IntellaTip MiFi XP catheter and granted 510(k) clearance for the Zurpaz 8.5 French steerable sheath for electrophysiology (EP) ablation procedures.
The idea that surgery to relieve the pressure caused by hemorrhaging in the brain is a perfect job for a robotic system is the basic premise of a new image-guided surgical system under development at Vanderbilt University. It employs steerable needles about the size of those used for biopsies to penetrate the brain with minimal damage and suction away the blood clot that has formed.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

August 20, 2013 — St. Jude Medical Inc. announced the acquisition of Endosense SA, a Switzerland-based company that has pioneered contact-force measurement in catheter ablation. The acquisition adds to the company’s electrophysiology portfolio and provides a robust platform for future product development.
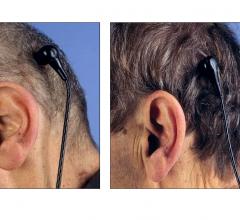
Cardiac surgeons and cardiologists at the University of Maryland Heart Center are part of a multi-center clinical trial evaluating the efficacy of powering heart pumps through a skull-based connector behind the ear. Typically, these devices for patients with severe heart failure are energized through an electrical cord connected at an abdominal site.

August 19, 2013 — Medtronic Inc. announced the submission of its first pre-market approval (PMA) module to the U.S. Food and Drug Administration (FDA) for the IN.PACT Admiral drug-eluting balloon.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Cook Medical is again shipping its Zilver PTX drug-eluting peripheral stent to medical centers in the United States, Japan, Europe and other major markets. The shipments follow a brief period of unavailability due to a voluntary recall by Cook related to an issue with the stent’s delivery catheter that has been resolved.
IMRIS Inc. announced that a craniotomy recently performed on a 5-year-old boy with epilepsy was the 500th neurosurgical case at Cook Children's Medical Center in Fort Worth, Texas, using its intraoperative magnetic resonance imaging (iMRI) system. Cook Children's was the second children's hospital in the United States and fourth hospital worldwide with an IMRIS system that features a high-field MRI, which moves between surgical and diagnostic rooms using ceiling-mounted rails.
Aplio 500 received top ranking in the Ultrasound – General Imaging category in the 2013 Best in KLAS Awards: Medical Equipment & Infrastructure.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
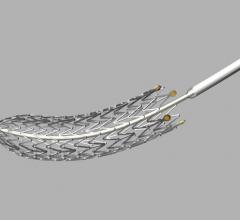
Patient enrollment has been initiated in a post-market registry for the Combo Dual Therapy Stent to evaluate its long-term safety and performance in routine clinical practice. The prospective, multicenter, all-comers REMEDEE Registry (Multicenter, Prospective, Clinical Outcomes After Deployment of the Abluminal Sirolimus Coated Bio-Engineered Stent — Combo Bio-Engineered Sirolimus Eluting Stent — Post Market Registry) will enroll 1,000 patients at nine European sites. As of today, more than 100 patients have already been enrolled.
Former President George W. Bush underwent heart surgery on the morning of Aug. 6, 2013, to receive a stent after an artery blockage was discovered during his annual physical. The routine physical included a stress test and an electrocardiogram. Abnormal results from the stress test prompted the former president to receive a computed tomography (CT) angiogram, and the surgery successfully took place one day after the blockage was discovered.
The first implantation of Tyrx Inc.’s new AigisRx R Fully Resorbable Antibacterial Envelope has taken place at the Vanderbilt Heart and Vascular Institute in Nashville, Tenn., by Christopher R. Ellis. The AigisRx R Antibacterial Envelope received U.S Food and Drug Administration (FDA) clearance on May 20, 2013.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Colibri Heart Valve LLC announced that enrollment its first-in-human feasibility study is continuing, and early clinical results from the second implantation of the Colibri device confirm the positive clinical findings observed in its first use. The Colibri transcatheter aortic valve implantation (TAVI) system is the first low profile, 14 French, pre-mounted, pre-crimped, and pre-packaged, ready-for-use TAVI system.
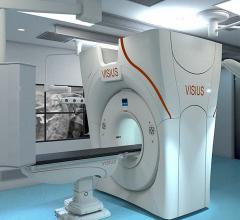
IMRIS Inc. has obtained regulatory CE mark for Visius iCT, the first and only ceiling-mounted intraoperative computed tomography (CT), allowing for sales and marketing in the European Union.
The William Stamps Farish Fund in Houston has donated $400,000 to a collaboration between the Institute for Computational Engineering and Sciences (ICES) at The University of Texas at Austin and the Texas Heart Institute (THI) to study life-threatening vulnerable plaques, the cause of at least two-thirds of all heart attacks, and new ways to prevent them.


 August 22, 2013
August 22, 2013