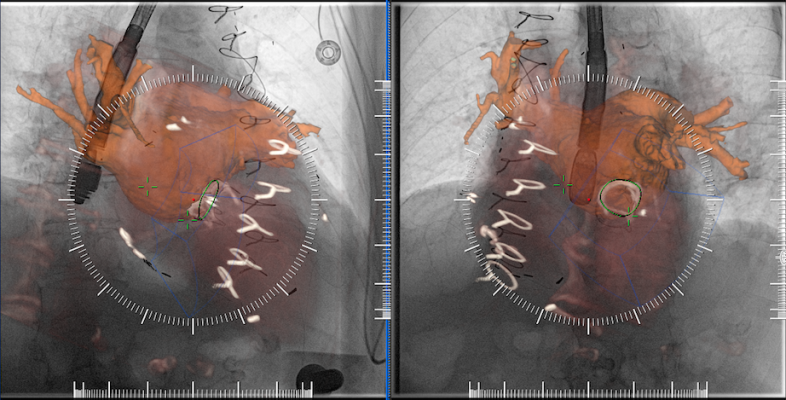
An example of multimodality image fusion with live angiography to enhance soft-tissue visualization during complex procedures. This example is from Siemen's new TrueFusion software released in 2018.
There are a few recent trends in X-ray angiography imaging systems that hospitals should be aware of if they are looking for new or replacement technology.
1. Technology to Lower Radiation Dose
As interventional procedures become more complex, imaging and procedural times naturally increase. To compensate for the higher imaging X-ray doses involved, vendors have developed a new generation of angiography systems that address the need to lower dose for both the operator and the patients. The current-generation systems offered by GE Healthcare, Canon, Philips, Shimadzu and Siemens all offer lower X-ray dose while preserving image quality. This has been accomplished with a combination of new X-ray tubes, more sensitive detectors, new image reconstruction software, image hold technologies, and by using better navigation and multimodality image fusion software to guide procedures.
The most recent example of this was in January 2019, with Philips Healthcare launching its Azurion 7 with FlexArm system. It is designed to enhance positioning flexibility for image-guided procedures. Due to increasingly complex interventions, clinicians need to quickly and easily visualize critical anatomy and identify changes to the patient during the procedure. This system includes technology to make this easier in both 2-D and 3-D. As the clinician moves the system, the image beam automatically maintains alignment with the patient, allowing more consistent visualization and enabling them to keep their focus on the treatment.
“With FlexArm, Philips’ engineers have overcome near-impossible geometric and mechanical barriers to enable clinicians to achieve clinical excellence in image-guided therapy,” said Barry T. Katzen, M.D., founder and chief medical executive of the Miami Cardiac and Vascular Institute, Baptist Health South Florida. “FlexArm enables us to dramatically optimize procedures around the patient: We can get the optimal view of what’s going on inside the patient without encumbering all of the clinicians that are working around the table. The result is an innovation that’s not only clinically important but also very simple and intuitive to use – a critical factor in the heat of a complex procedure.”
The Azurion with FlexArm’s design provides a high level of flexibility with movement on eight different axes, all controlled with its single controller. Simulation tests with clinicians demonstrated the potential to significantly reduce the repositioning of the patient, staff and equipment to improve access for minimally invasive procedures, including radial access.
The Philips Azurion 7 C20 with FlexArm has both CE mark and U.S. Food and Drug Administration (FDA) clearance.
2. Offering a Complete Cath Lab Solution
When a hospital installs a new interventional lab, many want a complete package so they do not have to subcontract with multiple vendors. A key element of the labs is integration with a hemodynamic system. All the major angiography vendors now partner with other vendors to offer complete solutions with hemodynamics.
The most recent partnership was Shimadzu Medical Systems USA and Change Healthcare partnering last fall, where Shimadzu will offer Change's Cardiology Hemo on its Trinias line of angiography systems. Trinias is a single-plane system available in floor or ceiling mounts or as a bi-plane mounted system.
3. New Echo Fusion Solutions for Procedural Navigation
Complex procedures, especially in the structural heart space, require visualization of the surrounding soft-tissue anatomy beyond what 2-D angiography can offer. Ultrasound is usually employed for complementary imaging during procedures, usually with transesophageal echo (TEE). A couple vendors have taken TEE a step further with integration with the live fluoro imaging to co-register the images and display them in one fused view.
There were two new product introductions in this space in 2018. Philips introduced the Epiq CVxi interventional cardiovascular ultrasound system. Specifically designed for use in the cath lab, the Epiq CVxi with EchoNavigator streamlines communication between the interventional cardiologist and the echocardiographer during complex interventional exams. Combining live ultrasound and X-ray information into one fused view, EchoNavigator helps interventional cardiologists oversee procedures along with the location of key anatomical structures. The technology uses Epiq's artificial intelligence to automatically identify and extract anatomical structures to speed the image fusion process and show outlines of the anatomy on live TEE or on fused fluoroscopy.
Siemens introduced its version of a TEE/angiography fusion system with its TrueFusion, released in 2018. It allows fusion of TEE, cardiac computed tomography angiography (CTA) and live fluoro to aid navigation in complex structural heart procedures.
4. Integration of Augmented Reality in the Cath Lab
A new technology that is already on the horizon to aid procedural navigation in the cath lab is augmented reality. The technology will enable operators to see true 3-D images of anatomy in a heads-up display while they are looking at the patient or at the main screen in the lab.
Philips Healthcare showed a conceptual work-in-progress of this technology at the Radiological Society of North America (RSNA) meeting in 2017, allowing attendees to put on the headset and manipulate 3-D images with hand movements in the air. NovaRad and TeraRecon have already commercialized similar augmented reality technology to aid in advanced visualization of patient 3-D datasets. Novarad's FDA-cleared product allows surgeons to fuse a dataset to a patient on a surgical table so they can scroll through the slices to find the exact locations they need to access in the underlying tissues.
In February 2018, the National Institutes of Health (NIH) awarded a $2.2 million research grant to the company SentiAR Inc. to advance its work to design augmented reality (AR) software to improve visualization in cardiac surgeries and interventional procedures. The grant was awarded following peer review by a panel of cardiologists, engineers and other experts. SentiAR will receive the funding in milestone installments as it advances its platform. It uses the Microsoft heads-up display, allowing physicians to view, measure and manipulate real-time holographic images of the patient’s heart during procedures while still being able to clearly see around the room.
Using real-time navigation data feeds rather than magnetic resonance imaging (MRI) or computed tomography (CT), the SentiAR intraprocedural solution provides clinicians with patient-specific anatomy in a holographic display, including catheter movement. The software is aimed at reducing operating time and radiation exposure to clinicians, and potentially improving outcomes for patients. In-human engineering testing began in the summer of 2017.
“Our goal is to provide physicians who perform cardiac ablation procedures with a patient-specific hologram of the heart and the instruments that they are using inside of it,” said Jennifer Silva, M.D., SentiAR chief medical officer. “By improving the visualization of this information and empowering the physician with direct control of the model, we will make these procedures both simpler and safer. ”
SentiAR was hoping to have its technology submitted for FDA review in 2019.
5. Imaged-based FFR May Replace Pressure Wires
In interventional cardiology, technology has been developed to replace pressure wires to assess fractional flow reserve (FFR) to determine if a lesion is negatively impacting hemodynamics and if stenting is required. This technology will likely soon be offered as an option on angiography imaging systems.
In December 2018, the FDA cleared CathWorks' FFRangio System. It derives the FFR numbers from routine X-rays acquired during a diagnostic angiogram procedure. It is noninvasive and performed intra-procedurally during coronary angiography, eliminating additional time and costs associated with invasive FFR. The system provides a 3-D reconstruction of the entire coronary tree with FFR values along each vessel.
The system demonstrated accuracy versus the invasive fractional flow reserve (FFR) wire in the FAST-FFR study. Read more about the trial in the article "FAST-FFR Trial of FFR-angio Technology Meets Primary Endpoint, Exceeded Performance Goals."
CathWorks also gained approval for a new Current Procedural Terminology (CPT) code, 0523T, for FFRangio last July.
Watch the VIDEO: Angiography Image-based FFR May Eliminate Need for Pressure Wires, an interview with William Fearon, M.D.
Pie Medical Imaging also has an FDA-cleared FFR-angio software called CAAS vFFR (Cardiovascular Angiographic Analysis Systems for vessel Fractional Flow Reserve). It can calculate the pressure drop and vFFR value in the coronary artery non-invasively, which means there is no need for a pressure wire and hyperemic agent.
Philips is partnering with HeartFlow to develop an FFR-angio system. Medis has developed its QFR solution that offers FFR-angio as part of its QAngio XA 3D solution. It has European CE mark, but doe not have FDA approval.
6. Use of Artificial Intelligence to Aid Navigation
There will be an increasing usage of artificial intelligence (AI) algorithms to speed automation on the backend of imaging systems and analysis software in the coming years. An example of this was shown at the 2018 Radiological Society of North America (RSNA) meeting. GE Healthcare showed its new Liver Assist Virtual Injection (VI) technology it developed for interventional radiology. It incorporates applied intelligence-enhanced software that identified the feeder blood vessels for liver tumors and identified the best locations to embolize the tumor. The AI also can enhance the blood to look like a contrast injection in the interventional lab to help map the vessels for procedural pre-planning prior to having the patient on the table. The software can show the direction of flow in each vessel segment and can overlay a vessel map onto 3-D rotational angiography images created in the cath lab to aid procedural navigation.
7. Patient Table Reduces Scatter Radiation in the Cath Lab
Along with the trend to lower X-ray dose during imaging are ways to reduce scatter radiation that showers operators and other lab staff with increased dose. In addition to mobile shielding and table-mounted lead skirts, a new patient table developed by Egg Medical, the EggNest cath lab patient table, showed a large reduction in scatter radiation in a study presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2018 meeting last fall.[1] The study showed the average scatter dose in various positions of the C-arm resulted in between 10,010 to 38,910 μSv/h. Standard shielding slightly reduced this to between 7,910 and 34,870 μSv/h. The EggNest system markedly reduced Total Room SR to 640 and 2,040 μSv/h.
Related Angiography System Content:
Recently Introduced Angiography Imaging Technology
VIDEO: Reducing Cath Lab Radiation Dose at Henry Ford Hospital
Defining the Cath Lab Workplace Radiation Safety Hazard
VIDEO: Strategies to Avoid Acute Kidney Injury Caused by Cath Lab Contrast
Dose-Lowering Practices for Cath Lab Angiography
VIDEO: Walk Around of a GE Healthcare IGS 7 Angiography System
VIDEO: Cath Lab Walk Through With a Philips Azurion at the University of Colorado Hospital
VIDEO: Angiography Image-based FFR May Eliminate Need for Pressure Wires — Interview with William Fearon, M.D.
VIDEO: Evolution of Transcatheter Mitral Valve Repair at the University of Colorado
VIDEO: The Evolution of Complex PCI at University of Colorado
14 Ways to Reduce Radiation Exposure in the Cath Lab
VIDEO: Technologies and Techniques to Reduce Radiation Dose in the Cardiac Cath Lab
References:


 October 24, 2025
October 24, 2025 









