Out-of-hospital cardiac arrest (OHCA) continues to be overwhelmingly fatal despite cardiopulmonary resuscitation (CPR) ...
Balloon Catheter
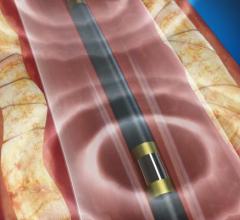
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
November 10, 2021 — Biosensors International Group Ltd., a developer and manufacturer of innovative medical devices ...
November 10, 2021 — Shockwave Medical a pioneer in the development of Intravascular Lithotripsy (IVL) to treat severely ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 10, 2021 — Shockwave Medical, a developer of Intravascular Lithotripsy (IVL) to treat complex calcified ...
November 10, 2021 — Philips Healthcare announced North American availability of new innovations in its portfolio of ...
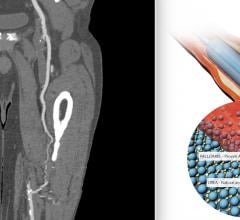
October 20, 2021 — 18-month results from the PRESTIGE* Below-the-Knee (BTK) study have been presented as a late-breaking ...
June 7, 2021 — A couple years ago a study showed a mortality safety signal in patients who underwent peripheral artery ...
May 20, 2021 — Boston Scientific Corporation announced it has initiated the AGENT IDE trial for the Agent Drug-Coated ...
This is a quick example of clinical use of the Shockwave Medical Intravascular Lithotripsy system that uses sonic wave ...
After covering cardiovascular technologies for 14 years, it is rare that I find a new technology that might actually be ...

The first devices developed for interventional cardiology were percutaneous transluminal coronary angioplasty (PTCA) ...
March 12, 2021 — Philips Healthcare announced the final, five-year results of two major randomized controlled trials ...

February 17, 2021 — The U.S. Food and Drug Administration (FDA) has cleared Shockwave Medical's Intravascular ...
November 13, 2020 — The new U.S. Food and Drug Administration (FDA) cleared XO Score system was successfully used to ...
November 3, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the Boston Scientific Ranger Drug-coated ...


 January 14, 2022
January 14, 2022