October 30, 2023 — Boston Scientific Corporation on Friday announced positive 12-month results from the pivotal AGENT ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
October 16, 2023 — A door-to-balloon (D2B) time of 90-minutes or less is associated with improved outcomes for heart ...
September 18, 2023 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & ...
September 14, 2023 — BIOTRONIK announced the two-year-results from the BIOLUX P-III BENELUX all-comers registry ...
September 11, 2023 — The first patient has been enrolled in a UK study of large vessel de novo coronary artery disease t ...
September 6, 2023 — The Cardiovascular Research Foundation (CRF) has announced the TCT 2023 late-breaking clinical ...
August 28, 2023 — Prasugrel monotherapy after percutaneous coronary intervention (PCI) with drug-eluting stents is not ...
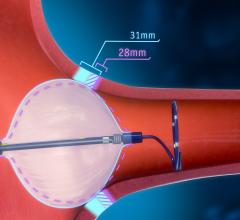
August 9, 2023 — Boston Scientific Corporation announced it has received U.S. Food and Drug Administration (FDA) ...
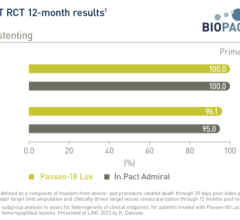
July 14, 2023 — BIOTRONIK announced the one-year subgroup results from the investigator-initiated BIOPACT randomized ...
May 30, 2023 — Twelve-month results from the SELUTION SFA trial have been presented for the first time at the Japan ...
May 30, 2023 — The US FDA, on the 24th of May 2023, granted an Investigational Device Exemption (IDE) approval for Conce ...
May 25, 2023 — Boston Scientific Corporation announced data supporting use of the company's key electrophysiology and ...
May 17, 2023 — Edwards Lifesciences announced that new data from the Benchmark Registry in Europe demonstrated the ...
May 12, 2023 — The US FDA has granted an Investigational Device Exemption (IDE) approval for Concept Medical Inc’s novel ...
May 4, 2023 — The first US patient has been enrolled in the SELUTION4SFA Sirolimus DEB study by Dr. Arthur Lee at the Ca ...


 October 30, 2023
October 30, 2023