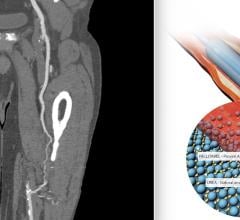
August 23, 2022 — The first US patient has been enrolled in the FDA SELUTION4BTK (Below-the-Knee) clinical trial ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
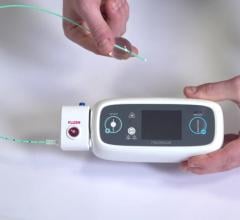
July 21, 2022 — Robocath, a company that designs, develops and commercializes innovative robotic platforms for the ...
July 12, 2022 — Biotronik announced the presentation of two studies on the performance of its drug-coated balloon ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
June 8, 2022 — The first patient has been enrolled in the FDA IDE BTK (Below-the-Knee) SELUTION4BTK clinical trial ...
June 2, 2022 — According to the U.S. Food and Drug Administration (FDA), Atrium Medical Corporation is recalling the iCa ...
Here is what you and your colleagues found to be most interesting in the field of cardiology during the month of May ...
May 27, 2022 — Medtronic today announced approval from the U.S. Food and Drug Administration (FDA) for the IN.PACT 018 ...
May 27, 2022 — The SELUTION SLR (Sustained Limus Release) is a novel sirolimus-eluting balloon that provides a ...
May 20, 2022 — New long-term data from the Safety Assessment of Femoropopliteal Endovascular Treatment With PAclitaxel ...
May 19, 2022 — One year outcomes from the Disrupt PAD III Trial comparing intravascular lithotripsy (IVL) with a drug ...
May 16, 2022 — Boston Scientific announced that Peter M. “Pete” Nicholas, co-founder and former CEO of Boston Scientific ...
May 13, 2022 — The MemorialCare Heart & Vascular Institute at Long Beach Medical Center has been designated the only ...
March 31, 2022 — In accordance with its commitment to patient safety, Medtronic recently voluntarily recalled a subset ...
Cardiovascular Systems, Inc., a medical device company developing and commercializing innovative interventional ...
February 15, 2022 – CERENOVUS, an emerging leader in neurovascular care and part of Johnson & Johnson Medical Devices ...

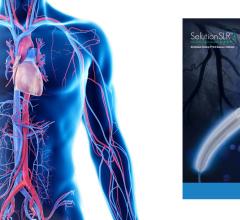
 August 23, 2022
August 23, 2022