May 31, 2019 — Terumo Medical Corp. is recalling the SoloPath Balloon Expandable TransFemoral System and Re-Collapsible ...
Catheters
May 21, 2019 – Medtronic plc announced its entrance into the guide extension catheter market with the global launch of ...
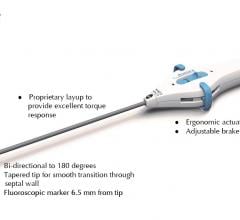
May 16, 2019 — BioCardia Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the Avance steerable ...
May 15, 2019 — Cordis, a Cardinal Health company, recently announced the full U.S. launch of its Radial 360 portfolio ...
April 10, 2019 — According to a new research study, straight tip guidewires, with a current share of more than a third ...
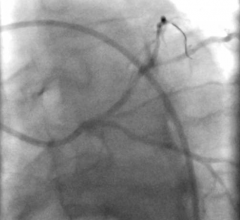
February 21, 2019 — Navitian, the new coronary microcatheter from iVascular, recently received CE mark approval. The ...
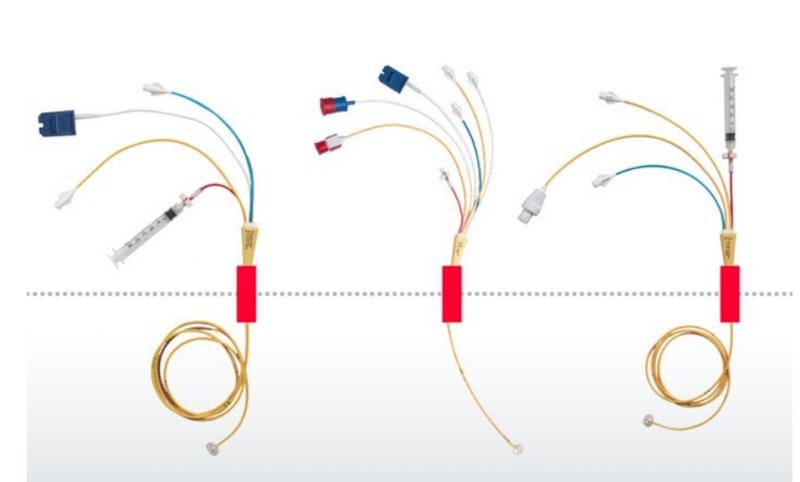
Edwards Lifesciences is recalling its 131F7, 131F7J, 131F7P, 131VF7P, 151F7 Swan-Ganz Thermodilution Catheters ...
January 31, 2019 — Cardiovascular Systems Inc. announced that the first patients in the United States were treated using ...
January 30, 2019 — XableCath Inc., announced that its XableCath blunt and abrasion tip catheters were cleared by the U.S ...
December 18, 2018 — Merit Medical Systems Inc. announced that it has acquired substantially all of the assets of ...
November 9, 2018 — VentureMed Group Inc. announced the initiation of a new study to determine if the Flex Dynamic ...
June 14, 2018 — The U.S. Food and Drug Administration (FDA) released the following two draft guidance documents:
- Coro ...
May 23, 2018 — Guerbet LLC USA announced the upcoming launch of SeQure and DraKon, two novel microcatheters for tumor ...
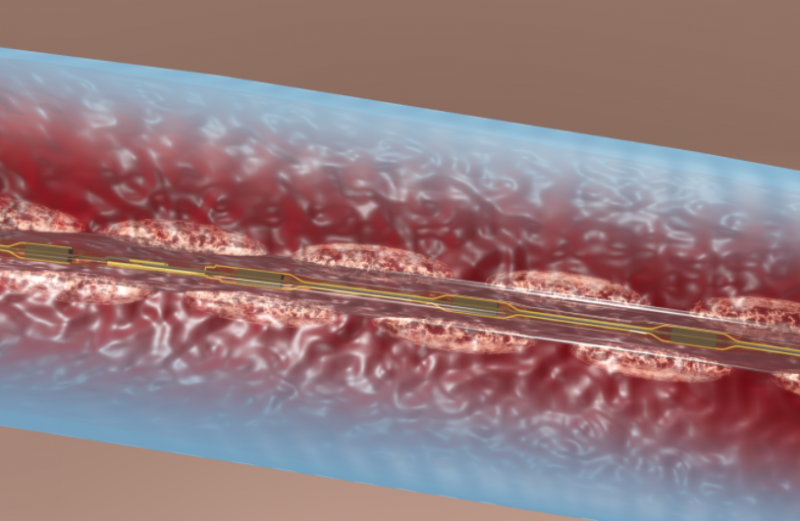
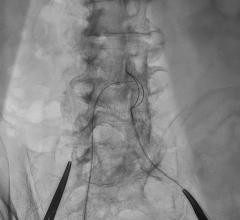
There are few downsides to using tibial venous access to treat deep vein thrombosis (DVT) with catheter-directed ...
January 8, 2018 —Guerbet announced that it has entered into an agreement under which it will acquire Israeli company ...


 May 31, 2019
May 31, 2019