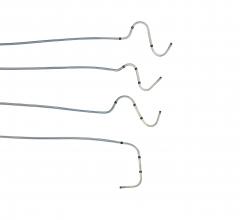
March 29, 2016 — St. Jude Medical Inc. announced CE Mark approval and launch of three new Quartet left ventricular (LV) ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
AliveCor, Inc., the leader in FDA-cleared electrocardiogram (ECG) technology for mobile devices, announced today the ...
March 22, 2016 — Electrophysiology company Acutus Medical announced that it has closed a $75 million Series C financing ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...

DAIC readers chose the following stories as the most popular content in 2015, based on website analytics. The list is ...
March 21, 2016 — BioSig Technologies Inc. announced it has signed an agreement to initiate development of its Pure EP ...
March 17, 2016 — Nearly half of all atrial fibrillation (AF) patients at the highest risk for stroke are not being ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
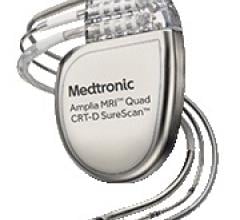
March 17, 2016 — Medtronic plc announced that it received CE (Conformité Européenne) Mark for the first and only cardiac ...
March 16, 2016 — The Valley Hospital in Ridgewood, N.J., is one of 15 U.S. sites currently enrolling patients in a ...
March 14, 2016 — Biotronik announced that its BioMonitor 2 heart monitor is now available for patients in the United ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
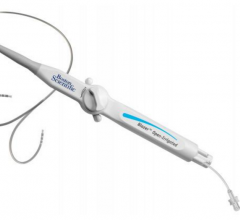
March 11, 2016 — Boston Scientific has received U.S. Food and Drug Administration (FDA) approval for the Blazer Open ...
March 10, 2016 — St. Jude Medical Inc. announced CE Mark approval for magnetic resonance (MR) conditional labeling for 1 ...
Managing inventory in the procedural area is often a challenge that creates substantial waste and inefficiency that ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
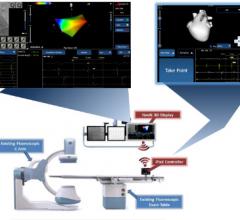
March 8, 2016 ― Fluoroscopy makes guiding a catheter through a blood vessel possible. However, fluoroscopy, a form of ...
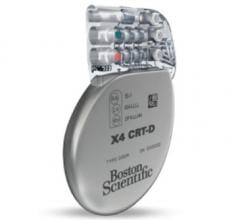
March 4, 2016 — Boston Scientific has received U.S. Food and Drug Administration (FDA) approval for the Acuity X4 ...
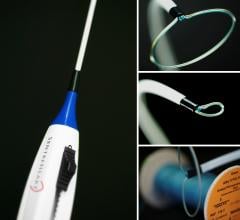
February 29, 2016 — APN Health LLC announced it received U.S. Food and Drug Administration (FDA) clearance to market ...

 March 29, 2016
March 29, 2016