June 10, 2022 — Setting a lower price to enter a competitive market with established brand leaders is a common ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).
May 17, 2022 — Heart Rhythm 2022 has come to a close, and the Heart Rhythm Society has released some stats regarding ...
Here are the top 10 performing videos on the Diagnostic and Interventional Cardiology (DAIC) website during the month of ...
May 2, 2022 – High-risk patients who need defibrillators to prevent cardiac arrest can experience fewer complications ...
Royal Philips, a global leader in health technology, announced late-breaking results of a large-scale, real-world ...
February 14, 2022 — Philips, a global leader in health technology, is supporting the American Heart Association’s multi ...

Children’s Hospital Los Angeles cardiologist Michael Silka, M.D., helped to pioneer the development of indications for ...
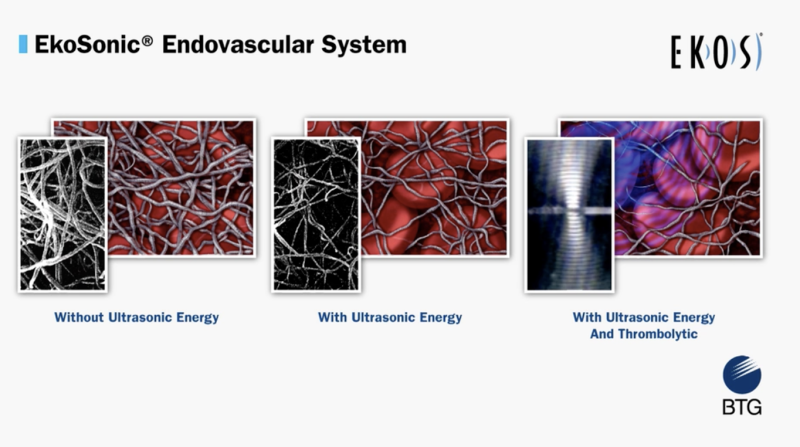
With 1.2 to 1.4 million new electrophysiology (EP) devices being prescribed to patients around the world each year ...
Khaldoun Tarakji, M.D., MPH, associate section head, cardiac electrophysiology, Heart and Vascular Institute at ...
Samir Saba, M.D., co-director of the University of Pittsburgh Medical Center Heart and Vascular Institute, and chief ...
July 28, 2021 — The U.S. Justice Department announced earlier this month that St. Jude Medical Inc. agreed to pay $27 ...
July 27, 2021 — Medtronic announced new data from the landmark WRAP-IT study published in Heart Rhythm,[1] demonstrating ...

Cardiovascular diseases are among the leading causes of death for people over 65 years old in North America and Europe ...
June 2, 2021 — Implicity, a leader in remote patient monitoring software and cardiac data management solutions ...
May 14, 2021 — The U.S. Food and Drug Administration (FDA) is advising patients and caregivers to keep any consumer ...


 June 10, 2022
June 10, 2022




![Medtronic announced new data from the landmark WRAP-IT study published in Heart Rhythm,[1] demonstrating a significantly lower infection risk for patients who develop hematomas after cardiac implantable electronic devices (CIEDs) when the Tyrx Absorbable Antibacterial Envelope is used at implant. The analysis showed an 82% reduction in major CIED infections among patients with the Tyrx Envelope who developed hematomas compared to patients in the control group who developed hematomas.](/sites/default/files/styles/content_feed_medium/public/X0000_Tyrx_AIGISRx_Envelope_with_Pacemaker.jpg?itok=BHsWJ9_y)

