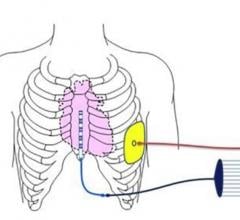
May 13, 2019 – A first-in-human pilot study of Medtronic's investigational Extravascular Implantable Cardioverter ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).
April 29, 2019 — Biotronik announced the full commercial launch of the Acticor device family, including Acticor DX and ...
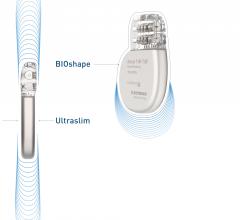
April 18, 2019 – Biotronik announced the European market release of what it calls the world’s smallest implantable ...

March 22, 2019 — The U.S. Food and Drug Administration (FDA) issued a safety communication to alert healthcare providers ...
Khaldoun Tarakji, M.D., MPH, associate section head, section of electrophysiology and pacing in the Robert and Suzanne ...

To extract or abandon broken or infected implantable, venous electrophysiology (EP) device leads has been a debate for ...
October 17, 2018 — The U.S. Food and Drug Administration (FDA) has reviewed information about potential cybersecurity ...
August 9, 2018 — Medtronic plc announced the start of a pilot study of its investigational Extravascular Implantable ...

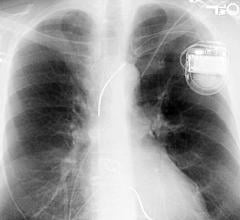
July 11, 2018 — Placement of implantable cardioverter defibrillators (ICDs) not meeting Centers for Medicare and ...
July 2, 2018 — University of Washington (UW) Medicine cardiologists have developed a tool to predict which heart failure ...
May 24, 2018 - Medtronic plc announced results from a research study demonstrating the feasibility of a novel approach ...
Here is an aggregation of all the news and late-breaking studies presented at the 2018 Heart Rhythm Society (HRS) Scient ...
May 10, 2018 – A new study is the first to test the clinical effectiveness of incremental peri-operative antibiotics as ...
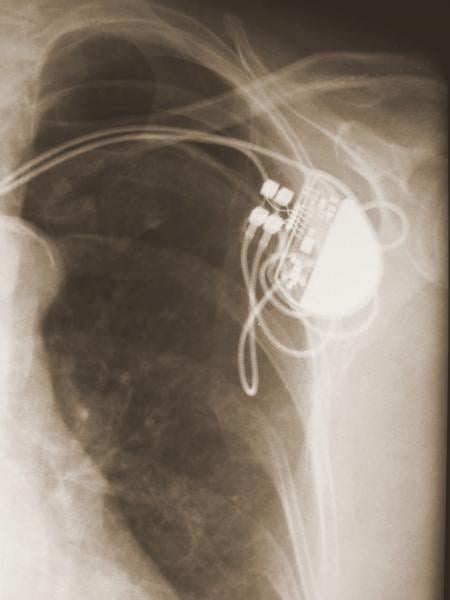
May 16, 2018 — The Heart Rhythm Society (HRS) released communication recommendations to assist healthcare professionals ...
May 16, 2018 — Boston Scientific announced results from an analysis of the LATITUDE database evaluating the successful ...

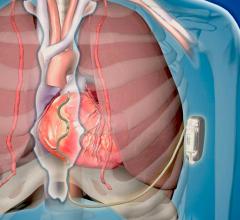
 May 13, 2019
May 13, 2019