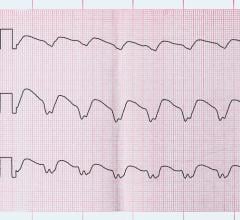
August 28, 2023 — Upgrade to cardiac resynchronization therapy with a defibrillator (CRT-D) reduces morbidity and ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).
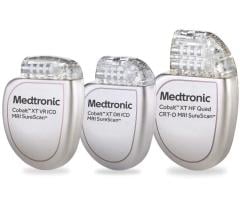
July 18, 2023 — The U.S. Food and Drug Administration (FDA) has issued a Class I recall on the Medtronic ICDs and CRT-Ds ...
June 14, 2023 — Vigorous exercise does not appear to increase the risk of death or life-threatening arrhythmia for ...
June 13, 2023 — A recent study highlights that implantable cardioverter-defibrillator (ICD) patients with new-onset ...
May 22, 2023 — Results from a pivotal clinical trial found a leadless pacemaker can deliver cardiac resynchronization ...
May 22, 2023 — Boston Scientific Corporation announced data supporting use of the company's key electrophysiology and ...

The Heart Rhythm Society (HRS) announced the full lineup of speakers and sessions, including late-breaking clinical ...
March 7, 2023 — Ninety-five percent of athletes with a diagnosed and treated genetic heart disease experienced no ...
February 17, 2023 — Medtronic plc has received CE (Conformité Européenne) Mark for the Aurora EV-ICD MRI SureScan ...
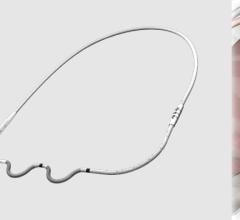
August 29, 2022 — Medtronic plc announced that its investigational EV ICD System – a first-of-its-kind defibrillator ...
August 19, 2022 — According to a release just issued by the U.S. Food and Drug Administration (FDA), Medtronic is ...
August 18, 2022 — A first-in-human multicenter trial involving Mayo Clinic used a new ablation technique for patients ...
August 4, 2022 — The Heart Rhythm Society (HRS) is a professional society representing a diverse population of ...
July 21, 2022 — The global automated external defibrillators market size was valued at USD 717 million in 2021. It is ...
July 13, 2022 — Adults with abnormal heart metabolism are up to three times more likely to experience life-threatening ...


 August 28, 2023
August 28, 2023