April 26, 2021 — Patients receiving an implantable cardioverter defibrillator (ICD) should be regularly screened for ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).
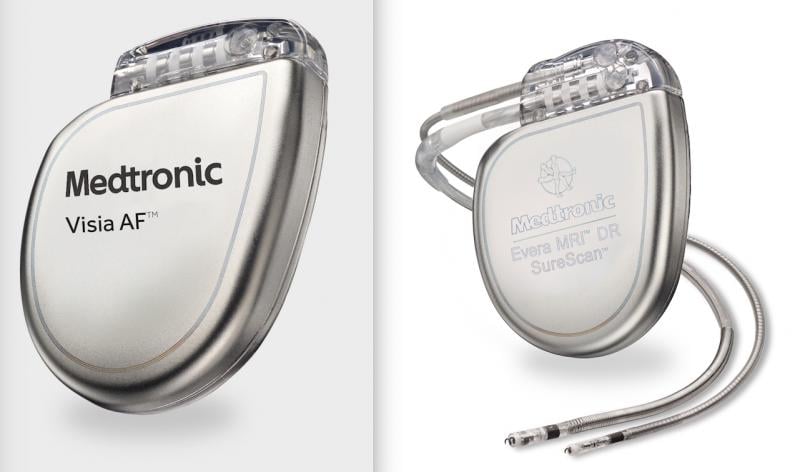
April 12, 2021 — Medtronic is recalling some of its implantable cardioverter defibrillators (ICD) and cardiac ...
April 2, 2021 — Today, the American College of Cardiology (ACC) launched the Electrophysiology (EP) Device Implant ...

A few weeks ago, a patient of mine experienced a cardiac event — his heart stopped. Completely. However, his implantable ...

February 2, 2020 — The U.S. Food and Drug Administration (FDA) has identified a recent recall of the Boston Scientific ...
February 2, 2021 – Optimize EP, a digital health company focused on transforming cardiac care by improving the current ...

February 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
January 8, 2020 — A new study published this week in HeartRhythm, the journal of the Heart Rhythm Society (HRS), found ...
Robert Kowal, M.D., chief medical officer of the Medtronic cardiac rhythm and heart failure division, said there has ...
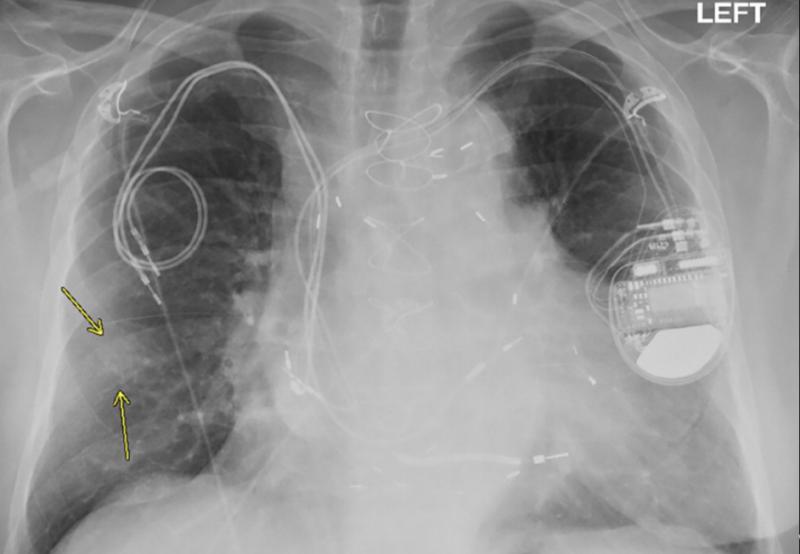
October 27, 2020 – Magnetic resonance imaging (MRI) examinations can be safely performed in patients with non-MR ...
September 8, 2020 - A new study presented at the 2020 European Society of Cardiology (ESC) Congress shows that Biotronik ...
July 13, 2020 - The U.S. Food and Drug Administration (FDA) has approved Abbott's next-generation Gallant implantable ...
Interview with Andrew D. Krahn, M.D., FHRS, head of the division of cardiology at St. Paul’s Hospital, and professor of ...
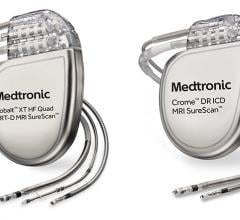
May 12, 2020 — Medtronic has received U.S. Food and Drug Administration (FDA) approval for its Cobalt and Crome ...
May 8, 2020 — Final results from the UNTOUCHED study of the Emblem Subcutaneous Implantable Defibrillator (S-ICD) System ...

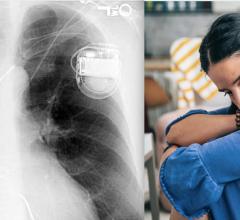
 April 26, 2021
April 26, 2021