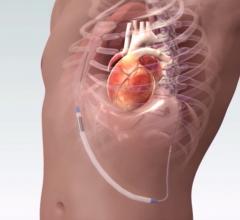
April 30, 2018 — LivaNova announced it completed the sale of its cardiac rhythm management (CRM) business to MicroPort ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).
April 13, 2018 — Medical advice about implanted cardiac defibrillators obtained via an online message board appears to ...
April 12, 2018 — Biotronik U.S. and Aziyo announced a strategic agreement allowing Biotronik to distribute Aziyo's ...
The Zoll LifeVest is a temporary, wearable defibrillator designed as a safety net for patients, especially those being ...
February 27, 2018 — Medical devices, including cardiovascular implantable electronic devices, could be at risk for ...
February 26, 2018 — The U.S. Food and Drug Administration (FDA) announced that Medtronic is recalling certain implantabl ...
January 3, 2018 — Abbott announced U.S. Food and Drug Administration (FDA) approval for magnetic resonance (MR) ...
Emanuel Kanal, M.D., director of MRI services and professor of radiology and neuroradiology at the University of ...
November 25, 2017 — Sitting in, or standing close to the charging port of a Tesla electric vehicle did not trigger a ...
October 30, 2017 — The American College of Cardiology, along with the American Heart Association and the Heart Rhythm ...
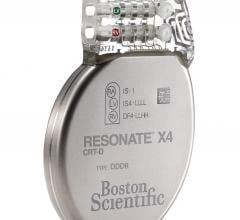
September 27, 2017 — Boston Scientific recently launched the Resonate family of implantable cardioverter defibrillator ...
September 22, 2017 — Abbott announced U.S. Food and Drug Administration (FDA) approval for magnetic resonance (MR)-condi ...
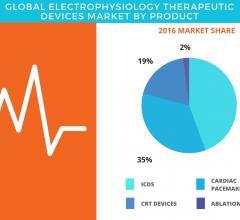
September 11, 2017 — According to the latest market study released by Technavio, the global electrophysiology ...

August 29, 2017 — The U.S. Food and Drug Administration (FDA) approved a firmware update that is now available to reduce ...
July 21, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and availability of the Intica DX ...


 April 30, 2018
April 30, 2018