May 8, 2020 — A new clinical trial is the first to compare the safety and efficacy of subcutaneous implantable ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).
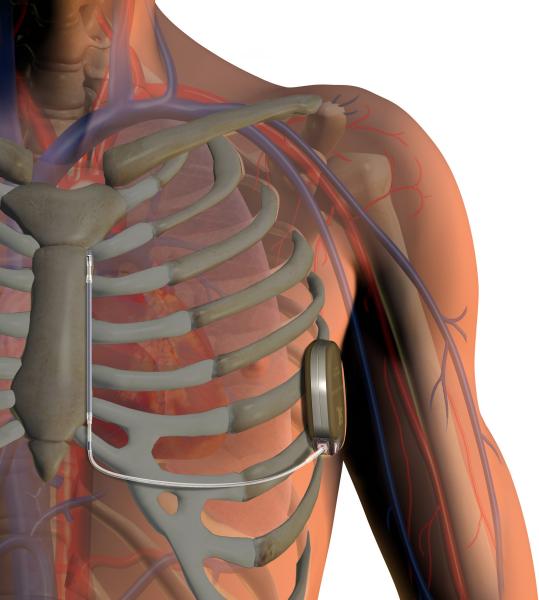

Cardiovascular disease is the leading cause of death for women in North America, and women with heart failure often ...
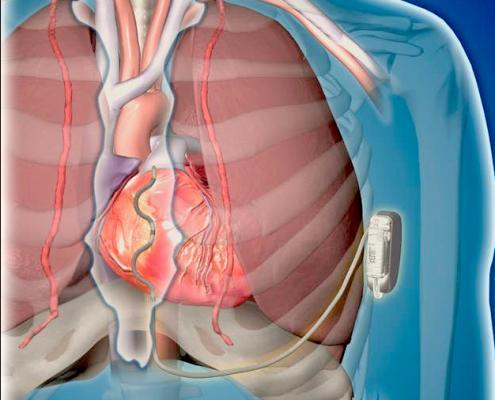
Cardiac rhythm management (CRM) devices in use today are evolving to raise the bar beyond monitoring and managing ...
November 6, 2019 — Cleveland Clinic announced the Top 10 Medical Innovations for 2020 at a multimedia presentation last ...
October 11, 2019 — Medtronic plc announced the start of a worldwide pivotal study evaluating its investigational Extrava ...
September 18, 2019 — Early use of an implantable cardioverter-defibrillator (ICD) after primary coronary intervention ...
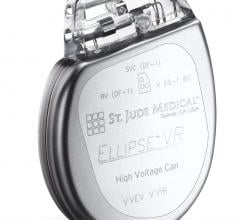
August 5, 2019 — Abbott is recalling the Ellipse Implantable Cardioverter Defibrillators (ICDs) because electrical ...
July 31, 2019 — The chances of patients experiencing complications after having a cardiac device implanted vary ...
For patients at risk for sudden cardiac arrest (SCA) who are being evaluated for a permanent implantable cardioverter ...

This is a brief overview of updates on implantable cardioverter defibrillators (ICD), including new technology ...
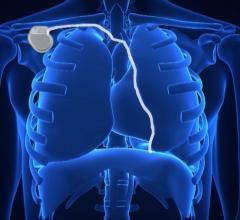
May 15, 2019 — A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The ...
May 15, 2019 - Three new studies show that patients who are medically indicated for implantable heart devices, including ...
May 14, 2019 – Results from new research show that passengers with cardiac implantable electronic devices (CIEDs), such ...
May 13, 2019 — Boston Scientific announced acute results from the UNTOUCHED study evaluating safety and efficacy of the ...
May 13, 2019 – Results from a new survey are the first to report a large discrepancy in patient’s knowledge of their ...

 May 08, 2020
May 08, 2020
![A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The new scoring system was presented as a follow up to that study during a late-breaking session at Heart Rhythm 2019, the Heart Rhythm Society's 40th Annual Scientific Sessions.](/sites/default/files/styles/content_feed_medium/public/PADIT_Infection_Risk_score.jpg?itok=O1-YAcMm)